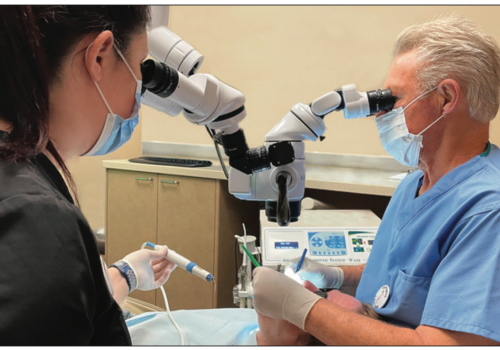
A significant problem in clinical dentistry in general, and in restorative dentistry and endodontics in particular, is isolation of the operative field for moisture control. The root canal system to be obturated must be dry in order to obtain a good seal, and contamination with blood must be avoided. During a direct pulp capping procedure, hemorrhage must be controlled. When attempting to seal a root perforation, a dry field is essential. Further, during apical surgery, the retro-preparation must be absolutely dry.
 |
| Figure 1. ProRoot MTA. |
Recently, Torabinejad and colleagues1 developed a new cement named Mineral Trioxide Aggregate (MTA; ProRoot MTA, DENTSPLY Tulsa Dental) (Figure 1), which appears to have all of the characteristics of an ideal cement to seal communication between the root canal system and the oral cavity (mechanical and carious pulp exposures), and between the root canal system and the periodontium (iatrogenic perforations, open apices, resorbed apices, root-end preparations).
MTA is an endodontic cement that is extremely biocompatible, capable of stimulating healing and osteogenesis, and is hydrophilic. MTA is a powder that consists of fine trioxides (tricalcium oxide, silicate oxide, bismute oxide) and other hydrophilic particles (tricalcium silicate and tricalcium aluminate, responsible for the chemical and physical properties of this aggregate2), which set in the presence of moisture. Hydration of the powder results in formation of a colloidal gel with a pH of 12.5. The gel solidifies to a hard solid in approximately 3 to 4 hours. This cement is different from other materials currently in use because of its biocompatibility, antibacterial properties, marginal adaptation and sealing properties, and its hydrophilic nature.1
In terms of biocompatibility, Koh et al3,4 and Pitt Ford et al5 demonstrated the absence of cytotoxicity when MTA came in contact with fibroblasts and osteoblasts, and the formation of dentin bridges when the material was used for direct pulp capping. Other studies 6-9 demonstrated the growth of cementum, periodontal ligament, and bone adjacent to MTA when used to seal perforations, as well as when employed as a retrofilling material in surgical endodontics.
Torabinejad et al10 demonstrated that the antibacterial properties of MTA are superior to that of amalgam, IRM (a zinc-oxide eugenol cement reinforced with polymethyl methacrylate), and SuperEBA (a zinc-oxide eugenol cement reinforced with aluminum oxide and with ethoxybenzoic acid). Nonetheless, its antimicrobial spectrum is limited, and if bacterial contamination is suspected or if acute inflammation is present, it is advisable to raise the pH and disinfect the root canal using a calcium hydroxide paste for one week before MTA.11 Furthermore, the marginal adaptation and sealing properties of MTA are far superior to amalgam, IRM, and SuperEBA.9,12-15
As noted, the characteristic that distinguishes MTA from other materials used to date in endodontics is its hydrophilic properties. Materials used to repair perforations, to seal the retro-preparation in surgical endodontics, to close open apices, or to protect the pulp in direct pulp capping are inevitably in contact with blood and other tissue fluids. Moisture may be an important factor due to its potential effects on the physical properties and sealing ability of the restorative materials.7 As shown by Torabinejad et al,7 MTA is the only material that is not affected by moisture or blood contamination; the presence or absence of blood seems not to affect the sealing ability of the mineral trioxide aggregate. In fact, MTA sets only in the presence of water.2
PULP CAPPING WITH MTA
Among the materials available today for direct pulp capping,5 MTA is the material of choice. Pulp capping is indicated for teeth with immature apices when the dental pulp is exposed and there are no signs of irreversible pulpitis.11 In such cases, the maintainance of pulp vitality is extremely important, and MTA is preferred to calcium hydroxide. Recent studies have shown that MTA stimulates dentin bridge formation adjacent to the dental pulp; dentinogenesis of MTA can be due to its sealing ability, biocompatibility, and alkalinity.5 Faraco and Holland16 demonstrated that in teeth treated with MTA, all bridges were morphologically tubular, and in some specimens the presence of a slight layer of necrotic pulp tissue was observed in the superficial portion of these bridges. This suggested that the material, similarly to calcium hydroxide, initially causes necrosis by coagulation in contact with pulp connective tissue. This reaction may occur because of the product’s high alkalinity, as the pH is 10.2 during manipulation and 12.5 after 3 hours.1 Holland et al6 demonstrated the presence of calcite crystals in contact with MTA implanted in rat subcutaneous tissue. Those calcite crystals attract fibronectin, which is responsible for cellular adhesion and differentiation. Therefore, it is believed that the MTA mechanism of action is similar to that of calcium hydroxide, but in addition, MTA provides a superior seal against bacteria.16
For those reasons, MTA is preferred to calcium hydroxide. Nevertheless, MTA has only recently been introduced, and no long-term studies on its efficacy have been published. Therefore, it is necessary to recall treated patients on a regular basis to determine if treatment has been successful or if root canal therapy is needed.
Operative Sequence for Pulp Capping
 |
 |
| Figure 2. Dovgan carriers. | Figure 3a. Preoperative radiograph of the mandibular right quadrant. The young patient is only 6-years-old and only the mesial cusps of the mandibular first molar are erupted. Caries is already present with a pulpal involvement. The tooth is completely asymptomatic. |
 |
 |
| Figure 3b. After removal of the caries, the pulp exposure was covered with MTA, a wet cotton pellet, and a temporary cement. | Figure 3c. Seven-month recall. |
 |
 |
| Figure 3d. Fifteen-month recall. | Figure 3e. Two-year recall. |
 |
| Figure 3f. Four-year recall. The tooth tests vital, is completely asymptomatic, and there is no evidence of calcification in the pulp chamber. |
After achieving anesthesia and isolation with a rubber dam, the exposed pulp is irrigated with sodium hypocholrite to control bleeding. The MTA powder is mixed with sterile water, and the mixture is placed in contact with the exposure using a Dovgan carrier (Figure 2). Compress the mixture against the exposure site with a moist cotton pellet. Place a moist cotton pellet over the MTA and fill the rest of the cavity with a temporary filling material. After 4 hours, the patient is seen again, the rubber dam is positioned, the temporary filling material and cotton are removed, and the set of the material is assessed. Then, the tooth can be restored (Figures 3a through 3f).
PERFORATION REPAIR WITH MTA
Recently, the prognosis of teeth with a perforation has improved with the use of the operating microscope17 and the introduction of MTA.18
When clinicians want to predictably repair a perforation, they face two challenges:19 (1) to establish hemostasis, and (2) to select a restorative material that is easy to use, seals well, does not resorb, and is biocompatible, supporting new tissue formation.
Generally, a barrier is created to achieve a dry field and prevent the extrusion of the restorative material. On the other hand, all of the restorative materials currently used (amalgam, Super EBA, IRM, composite resins) require a dry field and do not promote new tissue formation.
 |
 |
| Figure 4a. The screw post has caused a strip perforation of the mesial root of this mandibular left first molar. The furcal involvement is evident.Figure 4a. The screw post has caused a strip perforation of the mesial root of this mandibular left first molar. The furcal involvement is evident. | Figure 4b. After the removal of the screw posts, the distal and mesiolingual canals have been retreated and obturated with warm gutta-percha. The mesiobuccal canal has been obturated with warm gutta-percha to the level of the perforation. |
 |
 |
| Figure 4c. The mesiobuccal canal has now been filled with MTA from the perforation to the orifice. | Figure 4d. Two-year recall. |
For the above reasons, and primarily because it is hydrophilic, MTA can today be considered the ideal material to seal perforations. In fact, cementum has been shown to grow over MTA, allowing for normal attachment of the periodontal ligament.20 Furthermore, MTA doesn’t require a barrier, is not affected by moisture or blood contamination, and seals better than any other material in use today (Figures 4a through 4d).
Operative Sequence for Treatment of a Perforation
The operative sequence to treat a perforation of the root or of the floor of the pulp chamber is as follows:
•First visit
(1) isolation of the operative field with a rubber dam
(2) cleansing of the perforation site
(3) in case of bacterial contamination, application of calcium hydroxide for 1 week. If this step is performed, the patient goes home, then returns to resume steps 4 through 7 at the second visit.
(4) application of 2 to 3 mm of MTA
(5) radiograph to check the correct positioning of the material
(6) application of a small, wet cotton pellet in contact with MTA
(7) temporary cement
•Second visit
(1) after 24 hours, removal of temporary cement to check if MTA is set
(2) completion of therapy.
Criteria for Assessing Success
To be able to state that success has been achieved following treatment of a perforation, the treated tooth must meet the following requirements:21
(1) absence of symptoms, such as spontaneous pain or pain on palpation or percussion
(2) absence of excessive mobility
(3) absence of communication between the perforation and the oral cavity/gingival crevice
(4) absence of a fistula
(5) normal function
(6) absence of a radiolucency adjacent to the perforation
(7) thickness of the periodontal ligament adjacent to the obturating material no more than double the thickness of the adjacent ligament.
If even only one of these criteria is lacking, therapy is not successful.
IMMATURE PULPLESS TEETH
Despite the demonstrated clinical success of calcium hydroxide apexification, there are some disadvantages of this technique.22 The apical closure is unpredictable. The time necessary to achieve the final result is variable, and for adults, an acceptable result may never be achieved. The treatment time necessary for induced apical closure in pulpless teeth in humans has not been established.23,24 This therapy requires multiple appointments for either reapplication of calcium hydroxide or to check its presence inside the root canal, and the time interval between visits is at least 3 months. This may lead to loss of the coronal seal with consequent recontamination and exposure of the healing tissues to bacteria. In these cases, an acute exacerbation and delayed healing response may occur.
For these reasons, many clinicians advocated obturation of teeth with open apices without inducing a natural apical barrier.25-30 In fact, the concept of obturating teeth with immature apices without first inducing a natural apical barrier is not new; several investigators31-34 have likewise indicated that success is attainable with this approach, which does not require repeated applications of calcium hydroxide.
The apex of a tooth should be considered as a dynamic area, capable of self repair.33,35 Occasional instances of continued root growth and apical closure in the presence of a periapical pathology are explained on the basis of remnants of vital tissue in the area.36 However, a procedure that requires multiple appointments involving frequent dressing changes and instrumentation may cause injury to the local tissue.37,38
For all the above mentioned reasons, and taking into consideration the work of Koenigs et al39 and Roberts et al24 (who demonstrated the efficacy of tricalcium phosphate in inducing apical closure respectively in monkeys and in humans), Coviello and Brilliant25 suggested a one-appointment procedure for obturating permanent teeth with nonvital pulps and open apices, using tricalcium phosphate as an immediate apical barrier against which gutta-percha would be condensed. In their study, they found no statistical difference in the success rate comparing the multi-appointment and one-appointment techniques; they did not observe overfilling for teeth treated with the one-appointment technique; the procedure was faster; fewer radiographs were required; there was less discomfort for the patient; and the results were predictable.25
Buchanan40 in 1996 suggested the use of freeze-dried demineralized bone to be packed to the end of the immature root canal to create a one-visit biocompatible apical matrix. The use of an operating microscope in such cases was extremely helpful, as it allows the clinician to observe to the areas of the apex or bone graft matrix.
MTA has been suggested as an ideal material to promote the formation of an apical barrier in a one-visit procedure.11 According to recent studies, when compared to calcium hydroxide and to the osteogenic protein-1, MTA induced the same amount of apical hard tissue formation, and without an inflammatory response.28 Other studies demonstrated newly formed bone, periodontal ligament, and cementum in direct contact with MTA.7,8 Therefore, because it provides a good apical seal (better than was observed with amalgam, IRM, and Super EBA),7,9,13-15,41-45 its antimicrobial properties,10 biocompatibility,3,8,46-51 and hydrophilic properties, and taking into consideration the successful clinical cases reported in the literature,11,18,51-53 MTA should now be considered the material of choice for the apical barrier technique in the treatment of pulpless teeth with open apices.
Operative Sequence for Pulpless Teeth with Open Apices
After application of the rubber dam and preparation of an adequate access cavity, the root canal system should be cleansed with copious irrigation using sodium hypochlorite (which can be delivered ultrasonically for enhanced activation). The root canals require only minimum shaping, and because of their size and the thinness of the dentinal walls, they need to be cleansed more than shaped in order not to increase fragility.
To improve disinfection of the canals, Torabinejad11 suggests using an intracanal medication with calcium hydroxide for 1 week. After rinsing calcium hydroxide from the root canal with irrigation and drying with paper points, the MTA powder is mixed with saline or sterile water, and the mixture is carried to the apical area with the prefitted Dovgan carrier. MTA must be positioned exactly at the foramen, as the material must be in direct contact with periapical tissues. Overfilling should be avoided. In general, the resistance of the periapical tissues is enough to prevent overfilling; nevertheless, there is no contraindication to the use of a resorbable matrix (Collacote), against which MTA could be condensed to the apex. For this purpose, the prefitted Schilder pluggers, as well as paper points, can be used. The thickness of the apical plug must be 3 to 4 mm. In order not to have voids, the use of ultrasonics is suggested (while slightly condensing the MTA with the plugger, the assistant is asked to touch the plugger with the ultrasonic tip). After completion, the extension of the apical plug is checked radiographically. If the apical plug is not satisfactory, the MTA is removed via saline solution irrigation, and the filling procedure is repeated.
 |
 |
| Figure 5a. Preoperative radiograph of the maxillary left central incisor. The patient is 55-years-old and this tooth (with an open apex) has not responded to previous therapy with calcium hydroxide. | Figure 5b. Intraoperative film with the Dovgan carrier in place. |
 |
 |
| Figure 5c. Three millimeters of MTA have been positioned at the foramen to form the apical barrier. | Figure 5d. After the MTA is set, the thermoplastic gutta-percha has been used to obturate the root canal. |
When the radiographic appearance looks ideal, a wet paper point is placed in direct contact with the MTA, and the access cavity is closed with a temporary seal and allowed to set for 3 to 4 hours. At the next visit, the rubber dam is placed, the temporary seal and paper point are removed, the hardness of the material is checked, and then root canal therapy is completed by filling the root canal with warm gutta-percha (Figures 5a through 5d). If the canal walls appear to be thin and fragile, it has been suggested that the rest of the root canal be completely filled with adhesive composite resin (without using gutta-percha) to strengthen the root structure.22
As stated previously, the use of the operating microscope is essential in these cases. Furthermore, to facilitate the positioning of the material, the clinician can carry the dry powder to the site. Touching the MTA powder with a wet paper point will, by capillary action, provide the necessary hydration.
The apical barrier technique using MTA is indicated for adult patients with pulpless teeth and immature apices. Using the traditional technique that employs calcium hydroxide will not be effective for these cases. Further, patients may find the calcium hydroxide approach unacceptable due to the multiple visits that are required.
The same technique using MTA is also indicated in the young patient, only if the traditional access cavity will allow a perfect visualization of the apical foramen using the operating microscope. If this is not obtainable and a further removal of crown structure should be necessary, in this case the traditional technique with calcium hydroxide remains the treatment of choice.
ROOT END FILLING
Due to its sealing properties, biocompatibility, and hydrophilic nature, MTA is considered the best choice for a retrofilling material.1,7-15,18,43-46,50,54 Its handling characteristics are considered to be excellent. In particular, the material can be used in the presence of blood.54
Operative Sequence for Root End Filling
After the preparation of the root end has been completed with ultrasonic instruments, the MTA is placed with a carrier and gently compacted with a small plugger. The best instruments for this purpose include the use of amalgam carriers like the Messing gun (R. Chige, Inc), or the new Dovgan MTA carriers, which are straight, bendable, or prebent (Quality Aspirators).
The material should be kept relatively dry so it does not readily flow, yet moist enough to allow manipulation and a workable consistency. If the assistant touches the plugger with an ultrasonic tip during the placement process, voids are eliminated, the density of the fill is better, and radiopacity is increased.
 |
 |
| Figure 6a. Preoperative radiograph of the mandibular left first premolar. The previous surgical procedure is failing. | Figure 6b. A fistulous track is present. |
 |
 |
| Figure 6c. Postoperative radiograph after the surgical retreatment. The old amalgam has been removed and the retro-preparation has now been filled with MTA. | Figure 6d. The one-year recall is showing complete healing, with the lamina dura surrounding the end of the root. |
The working time of MTA is approximately 2 hours, and this eliminates problems related to rapid setting that accompanies other materials. Finishing of MTA is accomplished by simply carving away excess material with a spoon excavator to the level of the resected root end. The moisture necessary to achieve the final set is from the blood, which fills the crypt after surgery (Figures 6a through 6d).
CONCLUSION
Mineral Trioxide Aggregate (MTA) is a relatively new material that has become the material of choice for certain endodontic applications. This article has described those applications, including the operative sequence for specific procedures.
References
1. Torabinejad M, Hong CU, Mcdonald F, et al. Physical and chemical properties of a new root-end filling material. J Endod. 1995;21:349.
2. Lee SJ, Monsef M, Torabinejad M. Sealing ability of a mineral trioxide aggregate for repair of lateral root perforations. J Endod. 1993;19:541.
3. Koh ET, Mcdonald F, Pitt Ford TR, et al. Cellular response to mineral trioxide aggregate. J Endod. 1998;24:543.
4. Koh ET, Torabinejad M, Pitt Ford TR, et al. Mineral trioxide aggregate stimulates a biological response in human osteoblasts. J Biomed Mater Res. 1997;37:432.
5. Pitt Ford TR, Torabinejad M, Abedi HR, et al. Using mineral trioxide aggregate as a pulp-capping material. J Am Dent Assoc. 1996;127:1491.
6. Holland R, De Souza V, Nery MJ, et al. Reaction of rat connective tissue to implanted dentin tubes filled with mineral trioxide aggregate or calcium hydroxide. J Endod. 1999;25:161.
7. Torabinejad M, Higa RK, Mckendry DJ, et al. Dye leakage of four root-end filling materials: effects of blood contamination. J Endod. 1994;20:159.
8. Torabinejad M, Pitt Ford TR, Mckendry DJ, et al. Histologic assessment of mineral trioxide aggregate as a root-end filling in monkeys. J Endod. 1997;23:225.
9. Torabinejad M, Watson TF, Pitt Ford TR. Sealing ability of mineral trioxide aggregate when used as a root-end filling material. J Endod. 1993;19:591.
10. Torabinejad M, Hong CU, Pitt Ford TR, et al. Antibacterial effects of some root-end filling materials. J Endod. 1995;21:403.
11. Torabinejad M, Chivian N. Clinical applications of mineral trioxide aggregate. J Endod. 1999;25:197.
12. Adamo HL, Buruiana R, Schertzer L, et al. A comparison of MTA, SuperEBA, composite and amalgam as root-end filling materials using a bacterial microleakage model. Int Endod J. 1999;32:197.
13. Bates CF, Carnes DL, Del Rio CE. Longitudinal sealing ability of mineral trioxide aggregate as a root-end filling material. J Endod. 1996;22:575.
14. Fisher EJ, Arens DE, Miller CH. Bacterial leakage of mineral trioxide aggregate as compared with zinc-free amalgam, intermediate restorative material and SuperEBA as a root-end filling material. J Endod. 1998;24:176.
15. Wu MK, Kontakiotis EG, Wesselink PR. Long-term seal provided by some root-end filling materials. J Endod. 1998;24:557.
16. Faraco IM, Holland R. Response of the pulp of dogs to capping with mineral trioxide aggregate or a calcium hydroxide cement. Dent Traumatol. 2001;17:163.
17. Ruddle CJ. Endodontic perforation repair: using the surgical operating microscope. Dent Today. 1994;13:48.
18. Cantatore G, Castellucci A, Dell’agnola A, et al. Applicazioni cliniche dell’MTA. G It Endod. 2002;16:29.
19. Ruddle CJ. Retreatment of root canal system. CDA J. 1997;25(11):765-799.
20. Pitt Ford TR, Torabinejad M, Mckendry DJ, et al. Use of mineral trioxide aggregate for repair of furcal perforations. Oral Surg. 1995;79:756.
21. Stromberg T, Hasselgran G, Bergstedt H. Endodontic treatment of traumatic root perforations in man: a clinical and roentgenological follow-up study. Sven Tandlak Tidskr. 1972;65:457.
22. Hachmeister DR, Schindler WG, Walker III WA, et al. The sealing ability and retention characteristics of mineral trioxide aggregate in a model of apexification. J Endod. 2002;28:386.
23. Corpron RE, Dowson J. Pulpal therapy for the traumatized immature anterior tooth. J Mich Dent Assoc. 1970;52:224.
24. Roberts SC Jr, Brilliant JD. Tricalcium phosphate as an adjunct to apical closure in pulpless permanent teeth. J Endod. 1975;1:263.
25. Coviello J, Brilliant JD. A preliminary clinical study on the use of tricalcium phosphate as an apical barrier. J Endod. 1979;5:6.
26. Goodell GG, Mork TO, Hutter JW, et al. Linear dye penetration of a calcium phosphate cement apical barrier. J Endod. 1997;23:174.
27. Pitts DL, Jones JE, Oswald RJ. A histological comparison of calcium hydroxide plugs and dentin plugs used for the control of gutta-percha root canal filling material. J Endod. 1984;10:283.
28. Shabahang S, Torabinejad M, Boyne PP, et al. A comparative study of root-end induction using osteogenic protein-1, calcium hydroxide, and mineral trioxide aggregate in dogs. J Endod. 1999;25:1.
29. Schumacher JW, Rutledge RE. An alternative to apexification. J Endod. 1993;19:529.
30. Weisenseel JA, Hicks ML, Pelleu GB. Calcium hydroxide as an apical barrier. J Endod. 1987;13:1.
31. Duell RC. Conservative endodontic treatment of the open apex in three dimensions. Dent Clin North Am. 1973;17:125.
32. Friend LA. The treatment of immature teeth with nonvital pulps. J Br Endodont Soc. 1967;1:28.
33. Moodnik RM. Clinical correlations of the development of the root apex and surrounding structures. Oral Surg. 1963;16:600.
34. Stewart D. Root canal therapy in incisor teeth with open apices. Br Dent J. 1963;114:249.
35. Zeldow LL. Endodontic treatment of vital and nonvital immature teeth. NY State Dent J. 1967;33:327.
36. Bayirli GS. Traumatized maxillary central incisor. J Endod. 1975;1:35.
37. Torneck CD, Smith JS, Grindall P. Biologic effects of endodontic procedures on developing incisor teeth, 3. Oral Surg. 1973;35:532.
38. Torneck CD, Smith JS, Grindall P. Biologic effects of endodontic procedures on developing incisor teeth, 4. Oral Surg. 1973;35:541.
39. Koenigs JF, Heller AL, Brilliant JD, et al. Induced apical closure of permanent teeth in adult primates using a resorbable form of tricalcium phosphate ceramic. J Endod. 1975;1:102.
40. Buchanan LS. One-visit endodontics: a new model of reality. Dent Today. 1996;15(5):36.
41. Adamo HL, Buruiana R, Rosenberg PA, et al. Bacterial assay of coronal microleakage: MTA, SuperEBA, composite, amalgam retrofillings. J Endod. 1996;22:196(abstract 33).
42. Nakata TT, Bea KS, Baumgartner JC. Perforation repair comparing mineral trioxide aggregate and amalgam using an anaerobic bacterial leakage model. J Endod. 1998;24:184.
43. Tang HM, Morrow S