Loss of height and/or width of the alveolar ridge may result from a number of situations. Traumatic injuries may destroy not only teeth but a portion of the supporting alveolar bone as well. When tooth loss from periodontal or other disease processes occurs, resorption of the underlying bone often follows swiftly in its wake. Whatever the cause, any severely diminished alveolar ridge creates formidable restorative challenges for the dental practitioner. Even if enough bone remains to allow for implant placement, optimal aesthetic results may be unachievable.
To deal with these challenges, a variety of approaches to bone augmentation have been developed over the years. One that has recently gained attention is the application of distraction osteogenesis techniques to correct dentoalveolar defects. Originally developed in the 1960s by orthopedic surgeons, distraction osteogenesis in some cases has been used to successfully expand the soft tissue and alveolar bone. However, this approach requires that the patient undergo a lengthy and inconvenient set of procedures, and potential complications may include fractures of the transport and/or anchorage segments of the affected bone.
A more traditional approach to bone grafting is to collect autogenous bone shavings with the use of bone traps during osteotomy creation or harvest larger quantities of bone from the patient’s ramus, symphysis, tuberosity, exostoses, or one of a number of extraoral sites. Such bone brings to the graft site not only inorganic bone matrix but also osteocytes, osteoclasts, osteoblasts, and various proteins.1 The use of autogenous bone does have some drawbacks, however. Only small quantities can typically be retrieved from osteotomy sites, and harvest from other locations carries risks of mortality and morbidity, in addition to complicating the treatment plan.2
Alternatives to autogenous bone include a variety of allograft, xenograft, and alloplastic materials. Since alloplastic materials are not converted into bone, their uses are limited. Allograft and xeno-graft bone graft materials allow for osteoconduction, and longstanding concerns about their antigenicity and/or disease-transmission risks have faded as processing techniques and procedures have improved. However, confining and stabilizing the bone particles in larger defect sites has continued to limit allograft and xenograft use.3
One solution to the aforementioned limitation is to use titanium mesh material to confine allograft material and serve as a rigid template for the proposed ridge form. Shaping the titanium is a simple process that requires only a tapered fissure bur and a spatula. Proficiency can quickly be developed. After a healing period of 4 or 5 months, the titanium is removed in a second surgery, and the dimensions of the augmented ridge are evaluated. After the soft tissue heals, implants can be placed in a minimally invasive flapless procedure.
This article reports on a case in which a major defect in the anterior mandible was reconstituted using irradiated cancellous bone particles contained within a titanium mesh framework.
CASE STUDY
 |
 |
|
Figure 1. An accident had caused loss of the patient’s mandibular central incisors, lateral incisor, and cuspid, along with a significant portion of the alveolar ridge. |
Figure 2. A vestibular incision exposed the defective ridge. |
 |
 |
|
Figure 3. Titanium mesh was cut and shaped to fit over the ridge. |
Figure 4. Perforation of the cortical plate induces bleeding from the periosteum and medullary bone. |
 |
 |
|
Figure 5. A mixture of irradiated cancellous bone and platelet-rich protein was used to build up the defective ridge. |
Figure 6. The titanium mesh, screwed into position. |
 |
 |
|
Figure 7. Absorbable collagen membrane covered the titanium mesh. |
Figure 8. Excellent tissue healing was evident 3 months after the initial surgery. |
The patient, a 20-year-old female, had been the victim of an accident in which both of her mandibular central incisors had been avulsed, along with her left mandibular lateral incisor and cuspid. The trauma had also destroyed a substantial section of the alveolar bone at those sites (Figure 1).
The treatment plan called for building up the height of the mandibular anterior ridge by using irradiated cancellous allograft bone mixed with platelet-rich plasma (PRP). After healing, 4 implants would then be placed in the newly created bone.
Immediately before the surgery, approximately 15 cc of blood was obtained by using a butterfly catheter in the patient’s right antecubital fossa. To prepare the platelet-rich plasma, the blood was then centrifuged at 2,200 rpm for 6 minutes. The layer of PRP was pipetted out, and 0.15 mL of calcium chloride was added to help begin the coagulation cascade.
The patient was sedated, and local anesthesia with infiltration was administered. A vestibular incision was created in order to reduce the likelihood of a later dehiscence. Evaluation of the exposed ridge confirmed that approximately 7 mm of height and 9 mm of width had been lost in the wake of the patient’s accident (Figure 2).
A 3×1.5-mm piece of sterile TiMesh titanium mesh (Medtronic of Canada) was cut and shaped to fit within the defect while serving as a form to contain the bone-grafting material (Figure 3). Sites for screwing the TiMesh to the existing bone also were identified. The cortical plate was then perforated with a tapered fissure bur at several points to induce bleeding from the periosteum and medullary bone, an important source of osteogenic components (Figure 4). An adequate blood supply is an absolute prerequisite for successful bone grafting.
Approximately 2.5 grams of irradiated cancellous bone particles (Rocky Mountain Tissue Bank) were combined with approximately 1 cc of PRP, creating a heavy mixture. Allowing it to sit and congeal for approximately 5 minutes further increased its malleability. This material was then plastered onto the top and sides of the defective ridge (Figure 5). The precut piece of TiMesh was positioned over the bone-grafting material and secured labially with three 1.5-mm screws (2 in the vestibule and 1 higher up on the ridge; Figure 6). The entire TiMesh-covered ridge was covered with a large piece of Neomem absorbable collagen membrane (Citagenix; Figure 7), and the tissues were approximated. Ten days later the sutures were removed, and the patient was given a new temporary prosthesis that had been hollowed out to prevent any impingement on the underlying tissues. She was instructed not to wear it when chewing or otherwise applying any pressure to the surgical site.
 |
 |
|
Figure 9. After removal of the titanium mesh 3.5 months after grafting, the ridge shows ample restoration of width and height. |
Figure 10. Healed tissue 2 weeks after the mesh was removed from the newly augmented ridge. |
 |
 |
|
Figure 11. Impression pins in place on implants. |
Figure 12. Four healing collars were placed. |
 |
 |
|
Figure 13. The final abutments. |
Figure 14. The final restorations were tried in 7.5 months after the initial ridge augmentation surgery. |
 |
 |
|
Figure 15. The final restoration. |
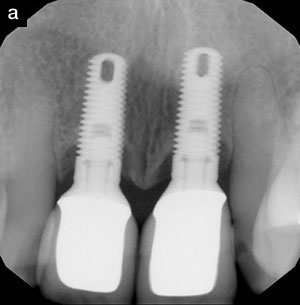
Figure 16. Radiograph of 3 of the 4 implants in the augmented ridge. |
Three months after the initial surgery, the tissues showed excellent healing (Figure 8), and palpation of the ridge suggested that the bone graft was integrating successfully. Although a small dehiscence had developed, exposing the TiMesh, the pa-tient reported no irritation to the soft tissue. Had the exposed metal bothered her, it would have been cut off with a tapered fissure bur and ground away.
Fourteen-and-a-half weeks after the initial surgery, a standard crestal incision was made and a mucoperiosteal flap was created, then a periosteal elevator and a scalpel were used to dissect the TiMesh away from the newly augmented ridge (Figure 9). Dexamethasone (Sandoz) was injected intraorally to help prevent inflammation, and the tissue was again approximated. Excellent healing was evident when the sutures were removed 2 weeks later (Figure 10).
Five months after the initial bone-grafting procedure, the patient returned for placement of one 3.5×16-mm and three 3.5×13-mm Groovy implants (Nobel Biocare). In order to avoid disturbing the blood supply further, these were placed through the mucosa (Figure 11). Healing collars were immediately connected (Figure 12), and the patient’s temporary prosthesis was modified to accommodate the healing collars while allowing for subtle loading.
Final impressions were taken 6 weeks later, and 3 weeks after that the final abutments were placed (Figure 13) and the final restorations tried in (Figure 14). Figure 15 shows the healed soft tissue around the final crowns. Figure 16 shows the positioning of the implants within the reconstructed ridge.
DISCUSSION
A longstanding concern about the use of allograft bone for grafting has been the possibility for exposing the patient to disease agents. However, proper processing of the bone has been demonstrated to virtually eliminate that danger. Reputable bone banks screen all donors for the presence of active infectious disease, malignancies, degenerative neurological disease, and any other diseases of unknown etiology. They also test blood samples for HIV 1 and 2, hepatitis B surface antigen, hepatitis C antibody, THLV 1 and 2, and syphilis, using FDA-licensed test kits.
All allograft material should be removed from the donor in an aseptic environment and stored in a frozen state until it is irradiated. The American Association of Tissue Banks recommends 1.5 megarads or greater to achieve adequate sterilization. (The bone particles used by the author are irradiated from a cobalt 60 source with between 2.5 and 3.8 mega-rads of radiation.)
For large bone defects where use of the titanium mesh support structure is appropriate, the author has found irradiated allograft bone to be a better graft material than bovine bone particulate, since the size of the allograft particles allows for a looser latticework in which infiltration of the new bone growth is more easily accomplished.
CONCLUSION
A number of approaches to grafting of maxillofacial bone require patients to undergo lengthy and expensive procedures that pose the risk of complications. The use of irradiated cancellous bone mixed with PRP and covered with a titanium framework avoids these problems and provides a successful and predictable means of regenerating lost alveolar ridges.
References
1. Smiler DG. Bone grafting: materials and modes of action. Pract Periodontics Aesthet Dent. 1996;8:413-416.
2. Kurz LT, Garfin SR, Booth RE Jr. Harvesting autogenous iliac bone grafts: a review of complications and techniques. Spine. 1989;14:1324-1331.
3. Vassos DM. Growing bone: clinical considerations. Oral Health. Sept 2001:31-52. Available at: http://www. oralhealthjournal.com/issues/table_of_contents.asp?PC=&RType=&issue=09012001. Accessed December 18, 2006.
Acknowledgment
The author gratefully acknowledges the assistance of Dr. Colin Diener, who completed the prosthetic restoration of this case.
Dr. Vassos is a Diplomate, American Board of Oral Implantology/Implant Dentistry, a Fellow and Diplomate, International Congress of Oral Implantology, and an Honored Fellow, American Academy of Implant Dentistry. He can be reached at (780) 488-1240.