INTRODUCTION
The millennium ushered in many new changes in dentistry, including minor tooth movement techniques for the general practitioner (GP). Recent advances have enabled doctors to predictably complete cases in nearly half the normal treatment time. This article will review the history, different techniques, and recent advances in accelerated orthodontics.
Dentistry has benefitted from a number of new advances during the past decade. One of the largest and most dynamic areas of growth is orthodontics. The introduction of Invisalign (in 1999) became a game changer for GPs. Prior to this, a GP would follow one of 3 paths related to orthodontics:
- The GP would express a devoted interest and pursue an orthodontic residency.
- The GP would express interest, preferring to remain in general practice and taking continuing education courses to gain orthodontic competence.
- The GP would simply refer all orthodontics to a local orthodontic specialist.
 |
| Figure 1. Cytokine cascade. |
 |
 |
| Figure 2. AcceleDent Aura with mouthpiece (courtesy of acceladent.com). |
Figure 3. SoftPulse Technology Illustration (courtesy of acceladent.com). |
I feel these options were largely the result of predoctoral orthodontic training in the United States. The sheer volume of material taught during dental school limited the time available to learn traditional orthodontic techniques. Another challenge was the ability of a predoctoral candidate to have the time necessary to treat a case from start to finish. Both the time to properly diagnose and treat a case, combined with the multiple administrative layers of clinical practice, made continual patient care very difficult, if not impossible.
Traditional orthodontic diagnosis, and an emphasis on mastering specific techniques, required a tremendous time commitment. Thankfully, dental schools have started shifting to competency-based education in predoctoral clinical orthodontics.1 The net result has been the ability for students and practicing GPs to perform some of the more simple cases involving minor tooth movement that were previously referred to orthodontic specialists.
The ease with which clear aligner therapy has been utilized to accomplish both diagnosis and treatment of minor tooth movement has been incredible. ClinCheck Software (Aligntech Institute) allows a doctor to accurately predict tooth movement as well as any challenging or stubborn areas.2 The ability to use the Align ClinCheck Software to visualize the final results is based on protocols developed with a database of more than a million cases, combined with rigorous lab testing using multiple force measurement apparatuses. The combination of these in vivo and in vitro analyses has resulted in increased confidence for practitioners.3
Just as with any procedure or technique in dentistry, proper training, case selection, and attention to diagnosis are keys to success. Also, starting with simple cases will act as a catalyst for excellent outcomes and patient satisfaction. The mantra crawl, walk, run applies to minor tooth movement, as it does for endodontics, oral surgery, or restorative procedures.
The past few years have seen an increased emphasis on reducing treatment times in orthodontics. The speed and velocity of tooth movement has been associated with potential iatrogenic external apical root resorption (EARR) of tooth roots.4-6 This phenomenon has not been observed with clear aligner treatment in more than a decade and a half, since Invisalign was introduced.7-9 I feel that the greatest reason for the lack of EARR with clear aligner therapy is simply due to the fact that, if the device is changed to a new one too quickly, the plastic deforms and the tooth will not “track” properly. This potential “excess velocity safety mechanism” has led to the consideration of alternative methods to orthodontically move teeth quicker.
Interestingly, the concept of accelerated tooth movement has developed on a parallel path with clear aligner therapy. Although the idea of speeding up tooth movement is not new, many new methods have been developed to accomplish this with reduced deleterious effects.10,11 A nice analogy might be to consider teeth the equivalent of fence posts in the ground. It would be very difficult to drag a fence post through solid concrete. But it would be easier to move it through soft earth, and even easier to move the post through wet concrete. How does one turn cured concrete into a softer substrate?
Let’s consider the standard forces associated with moving a tooth through jawbone. When a tooth has force placed on it, mechanically induced bone remodeling occurs. Bone is resorbed (via osteoclasts) on the compressive side and laid down (via osteoblasts) on the tension side of the tooth. Norton and Burstone12 and Kusy and Tulloch13 describe 3 distinct phases of force:
- Initial or strain phase. Occurs during a very short time period when the initial force is applied.
- Lag phase. Hyalinization tissue forms during this 2- to 3-week period, with little or no tooth movement.
- Post-lag phase. Tooth movement rapidly occurs as the hyalinized layer is removed, and the bone undergoes resorption via osteoclast action.
Oppenheim14 and Schwarz15 performed studies determining that the optimal tooth movement force is equivalent to capillary pulse pressure—approximately 20 to 26 g/cm2 of root surface.
Armed with this information, a number of new approaches have been developed to move our “fence posts” through a softer substrate while honoring Burstone’s time-proven force model. All these methods rely on a similar common theme known as a regional acceleratory phenomenon (RAP).16 Again, the idea is simple: use light mechanic forces on the tooth to move it through “softer bone” (Figure 1).17,18
Let’s now review of the different modalities associated with accelerated orthodontics.
CYCLIC FORCES (VIBRATION)
The principle behind the use of a vibratory method is to place light, alternating forces on the teeth via mechanical or electromagnetic pulses called AcceleDent SoftPulse Technology (AcceleDent). This results in a reportedly reduced second (lag) phase of force promoting greater movement and reduced potential resorption (EARR).19,20
The AcceleDent system, FDA approved and first introduced in 2009, focuses on the application of low magnitude cyclic forces by having the patient bite on a rubber interdental bite surface for 20 minutes each day. The device is relatively easy to use, but it has a steep cost component ($1,200, at press time) and relies heavily on dedicated patient compliance (a potential challenge). The device is lightweight, rechargeable, and has the ability to download patient use data to a computer (Figures 2 and 3).21
PHARMACEUTICAL ENHANCED TOOTH MOVEMENT
Many different forms of pharmacotherapy are being evaluated to enhance tooth movement, including injections of vitamin C and D metabolytes,22 calcitrol,23 corticosteroids, and many other compounds.24-26 This ongoing area of research deserves attention as these advances may become a routine part of treatment.
LIGHTWAVE ACCELERATED TOOTH MOVEMENT
OrthoPulse Light Accelerated Orthodontics
Biolux Research, located in Vancouver, Canada, originally developed a product to accelerate bone regeneration after extractions and healing for implant placement. Recently, the company has been completing clinical trials for a product developed for orthodontics called OrthoPulse. Both products rely on the use of near-infrared light emitting diode (LED) therapy or photobiomodulation to accomplish accelerated bone remodeling. The photobiomodulation mechanism involves using Cytochrome c oxidase present in the cell mitochondria to absorb OsseoPulse photons and convert the electromagnetic force energy into chemical energy (ATP).27,28
The OsseoPulse system involves wearing a device that fits over the patient’s head, similar to a telephone headset. Light is delivered from an LED source extraorally to the site requiring treatment for extractions and implants.
The Biolux Company saw benefit for this technology in accelerating orthodontic movement and developed an intraoral version of the device. The design is reminiscent of a football mouthguard, working with both traditional and clear aligner orthodontic therapy (Figure 4).
Photobiomodulation has seen significant clinical research throughout the years,29-32 including pharma-induced combination treatment.33 Loos et al34 are conducting clinical trials to evaluate efficiency and effectiveness of this exciting technology.
Laser-Assisted Accelerated Tooth Movement
Another recent area of interest is the use of laser energy in enhancing tooth movement. Although this area has seen significant lab research during the past decade, no viable commercial clinical system is presently available to the dental community (to the best knowledge of this author).35-38 I expect significant advances in the area of low-energy laser irradiation in the coming years (Figure 5).
DECORTICATION TECHNIQUES
Probably the most well-known methods of accelerating tooth movement are decortication techniques. This involves creating an iatrogenic insult in the bone/periodontal area near the teeth to be moved. The bone is subjected to mild trauma, resulting in a weakened condition that produces a transient osteopenia (bone healing). In essence, the bone area near the trauma “softens” (just like our fence post analogy), allowing the teeth to move through a less resistant substrate.39,40
 |
| Figures 4a to 4c. OrthoPulse device (courtesy of bioluxresearch.com). (Note from manufacturer: While not currently for sale in the United States and not yet having FDA approval, US and international clinical trials are underway for the OrthoPulse device.) |
 |
| Figure 5. AOO via laser therapy. |
Wilckodontics
Pioneered by orthodontist Thomas and periodontist William Wilcko, accelerated osteogenic orthodontics (AOO) was first introduced in 1998. AOO, often referred to as Wilckodontics41-45 usually involves performing a periodontal flap procedure followed by incisions or perforations in the bone between the teeth (Figure 6).
AOO is a fairly invasive procedure and has been primarily performed when a periodontal component of treatment is indicated. Osteotomies performed via the Wilcko technique generally involve incisions in the cortical bone along the long axis between the teeth and below the tooth apex. A handpiece and bur are used with copious amounts of irrigation. Manual instruments may be employed but lack the precise control of motor driven instrumentation. Microsaws are also used with the Wilcko technique. Treatment times have been reported to be twice as quick as normal orthodontic treatment.
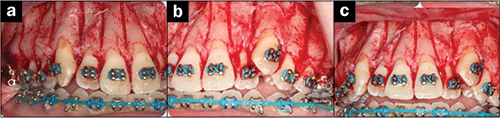 |
| Figures 6a to 6c. Corticotomies via Wilckodontic Technique (courtesy of wilckodontics.com). |
 |
| Figures 7a to 8. Pezio micro incision technique through tissue. The markers in the left photo identify where the piezocisions are to be made (courtesy of scielo.cl). |
Piezocision
Piezocision is a fairly new technique for performing osteotomies with accelerated orthodontics.46-50 The incisions follow a similar pattern to the Wilcko technique, but a piezosurgical device is used instead of a handpiece and bur. The piezo device uses ultrasonics with a modulated frequency and precise control of the vibrations at the device tip. The piezosurgical device operates along the same principles of the Stryker saw of a Cavitron (DENTSPLY Professional) unit. Advantages of a piezo device over rotary instrumentation include less heat, vibration, collateral tissue damage, pressure, and bleeding. Operators also report better control and precision when using piezosurgical versus rotary instrumentation (Figure 7).
Researchers are now evaluating the surgical results of piezocision through the soft tissue, or via microincisions without performing a periodontal flap procedure (Figure 8).51
Microperforation Technique in Accelerated Orthodontics
The most popular and promising advance in accelerated orthodontics for the GP involves microperforation with the Propel (Propel Orthodontics) device.
The Propel device utilizes similar physiologic science as the other 2 surgical methods listed above without the extreme invasiveness found with flap surgery or even microincisions. Like the others, Propel utilizes the RAP phenomenon to create a localized area of trauma, which in turn begins bone remodeling via a process known as cytokine expression.52 Simply put, these cytokine chemical messengers allow the surface of the osteoblasts to undergo receptor activation of nuclear factor-kappa ligand gene expression (also known as RANKL). Once again, our fence posts can now move through softer quicksand instead of hard concrete.53
 |
| Figure 9. Original Propel Excellerator device. |
 |
| Figures 10a to 10e. Propel (Propel Orthodontics) device in action. |
The technique for the Propel device requires no surgical instrumentation and can be performed in a standard dental operatory employing traditional aseptic protocol. The practitioner simply places very small microperforations in the cortical bone, directly through the gingival tissue. This microperforation of the cortical plate through the gingival soft tissue has been referred to as alveocentesis.54 Generally 2 or 3 perforations are placed between each tooth and may be limited to teeth that require challenging movements or an entire arch.55
An area of localized inflammation occurs in the peripheral tissues surrounding each perforation, resulting in bone remodeling. Because the perforations are one to 2 mm in diameter, this minimally invasive technique causes only mild patient discomfort. The cost is about $120 per disposable device (Figure 9).
The Propel device is a single-use, sterile, disposable manual perforator similar in size to a small handheld screwdriver. The device tip is pointed surgical stainless steel, 1.6 mm in diameter at its widest aspect, with a usable length up to 7.0 mm. The device has a protective sleeve that allows the practitioner to preset depths at one, 3.0, 5.0, or 7.0 mm.
Propel’s 1st generation device (Figure 9) had a plastic handle that was designed to be held in the palm of the hand, with the thumb and one or 2 fingers holding and twisting the shaft to the preset depth (Figure 10).
A 2nd generation Propel device was recently introduced that has a heavier, balanced metal handle and disposable screw tips. I have found the 2nd generation Propel device to be much more user friendly with less plastic waste. The metal handle, slightly wider than a dental handpiece, fits comfortably in the user’s palm and provides better heft for placing the perforations. Another significant improvement is the redesigned sleeve over the tip, which now rotates freely, preventing binding of the gingival tissue (Figure 11).
CASE EXAMPLES
Case 1
Invisalign and Propel—Two Propel applications were performed at the beginning and 10 weeks into treatment. Twenty-four aligners were used at 11-day intervals, no refinement, and total treatment time was 9 months. Two acetaminophen tablets were taken for discomfort at the time of Propel treatment and none the next day. Patient was comfortable a day later (Figures 12 and 13).
Case 2
Invisalign and Propel—Three Propel applications were performed, one at the beginning then at 12-week intervals for the next 2 applications. Thirty-three aligners were used at a 9-day interval. Four refinement aligners were used. Total treatment time was 11.5 months. Two acetaminophen tablets were taken for discomfort at the time of Propel treatment and none the next day (Figures 14 and 15).
The patients presented in both cases were thrilled with their new smile and to have completed treatment in significantly less time than originally indicated. (Cases and photos courtesy Dr. Ben Miraglia.)
 |
| Figure 11. Propel Excellerator RT. |
 |
| Figures 12a to 12e. Case 1, pre-op. |
 |
| Figures 13a to 13e. Case 1, post-op. (Case and photos courtesy Dr. Ben Miraglia.) |
 |
| Figures 14a to 14e. Case 2, pre-op. |
 |
| Figures 15a to 15e. Case 2, post-op. (Case and photos courtesy Dr. Ben Miraglia.) |
CLOSING COMMENTS
Like other acceleratory orthodontic techniques, micro-osteoperforation is relatively new to dentistry. As with any dynamic product or technique in the early adoption phase of growth, questions abound concerning safety and efficacy. I will address some of these concerns below.
 |
How does a GP introduce the Propel technique to a potential patient?
As with any dental procedure, proper training and informed consent are necessary before starting, including all risks and benefits. A discussion should include discomfort associated with the mildly invasive “dimpling” procedure and possible side effects. The tradeoff for decreased treatment time and/or better clinical results should be reviewed with the patient.
What if a micro-osteoperforation is too close or violates the periodontal ligament space?
Presently there is very little literature available concerning damage to the PDL during micro-osteoperforation. Discussion with clinical early adopters reveals that these insults heal without complication.48 More definitive research is necessary to support this anecdotal information.
How do you manage patient discomfort post-treatment?
Only acetaminophen or paracetomol (Tylenol) analgesics are indicated for patient discomfort. If a more potent medication is indicated, an acetomenaphin/oxycodone combination is recommended (Percocet). Standard nonsteroidal anti-inflammatory drugs (NSAIDs) are contraindicated for using as pain control because the mechanism of action is to reduce inflammation, a key component in any of the decortication techniques. Acetaminophen (or paracetamol) works by blocking chemicals that send pain messages and helps to cool the body. Ibuprofen, such as Advil and Motrin, is an NSAID that stops the body’s production of pain-causing chemicals and reduces fever and swelling. Acetaminophen is not an NSAID and, as such, will not inhibit the acceleratory orthodontic process.
What is the likelihood of post-op infection?
Because the Propel procedure is minimally invasive (compared to the other techniques described), no antibiotics are indicated.
Are there any contraindications to performing micro-osteoperforation procedures?
As with any dental procedure, a full medical history is indicated (with a follow-up consultation with the patient’s physician for any noted concerns). Other contraindications may include (not a complete list):
- Patients with bleeding or immune disorders
- Patients with high anxiety
- Patients requiring prophylactic antibiotics
- Patients taking or with a history of bisphosphonate use
- Patients taking anti-inflammatories
- Patients who have had previous radiotherapy of the jaw.
In Conclusion
Numerous new methods of acceleratory orthodontic treatment have been introduced recently (Table). More research is necessary to substantiate claims and enhance technology and techniques. The new Propel device used in conjunction with clear aligner therapy offers an efficient and predictable method of acceleratory orthodontic treatment for the GP.
References
- Oesterle LJ, Belanger GK. Orthodontic competency in predoctoral education in American dental schools. Eur J Dent Educ. 1998;2:14-18.
- ClinCheck software, Tooth Movement Assessment. San Jose, CA: Align Technology; 2010.
- ClinCheck software, Practice Protocols. San Jose, CA: Align Technology; 2010.
- Jung YH, Cho BH. External root resorption after orthodontic treatment: a study of contributing factors. Imaging Sci Dent. 2011;41:17-21.
- Brezniak N, Wasserstein A. Root resorption after orthodontic treatment: Part 1. Literature review. Am J Orthod Dentofacial Orthop. 1993;103:62-66.
- Abuabara A. Biomechanical aspects of external root resorption in orthodontic therapy. Med Oral Patol Oral Cir Bucal. 2007;12:E610-E613.
- Fowler B. A Comparison of Root Resorption Between Invisalign Treatment and Contemporary Orthodontic Treatment [master’s thesis]. Los Angeles, CA: University of Southern California; May 2010.
- Boyd RL. Periodontal and restorative considerations with clear aligner treatment to establish a more favorable restorative environment. Compend Contin Educ Dent. 2009;30:280-288.
- Krieger E, Drechsler T, Schmidtmann I, et al. Apical root resorption during orthodontic treatment with aligners? A retrospective radiometric study. Head Face Med. 2013;9:21.
- Proffit WR, Fields HW Jr, Sarver DM. Contemporary Orthodontics. 4th ed. St. Louis, MO: Mosby Elsevier; 2007:331-340.
- Wahl N. Orthodontics in 3 millennia. Chapter 2: entering the modern era. Am J Orthod Dentofacial Orthop. 2005;127:510-515.
- Norton LA, Burstone CJ. The Biology of Tooth Movement. Boca Raton, FL: CRC Press; 1988.
- Kusy RP, Tulloch JF. Analysis of moment/force ratios in the mechanics of tooth movement. Am J Orthod Dentofacial Orthop. 1986;90:127-131.
- Oppenheim A. Tissue changes, particularly of the bone, incident to tooth movement. Eur J Orthod. 2007;29(suppl 1):i2-i15.
- Schwarz AM. Tissue changes incidental to orthodontic tooth movement. International Journal of Orthodontia, Oral Surgery and Radiography. 1932;18:331-352.
- Melsen B. Biological reaction of alveolar bone to orthodontic tooth movement. Angle Orthod. 1999;69:151-158.
- Tyrovola JB, Spyropoulos MN, Makou M, et al. Root resorption and the OPG/RANKL/RANK system: a mini review. J Oral Sci. 2008;50:367-376.
- Baud’huin M, Duplomb L, Ruiz Velasco C, et al. Key roles of the OPG-RANK-RANKL system in bone oncology. Expert Rev Anticancer Ther. 2007;7:221-232.
- Bosio JA, Liu D. Moving teeth faster, better and painless. Is it possible? Dental Press J Orthod. 2010;15:14-17.
- Kau CH, Jennifer TN, Jeryl D. The clinical evaluation of a novel cyclical-force generating device in orthodontics. Orthodontic Practice US. 2010;1:43-44.
- Website information. Bellaire, TX: OrthoAccel Technologies.
- Collins MK, Sinclair PM. The local use of vitamin D to increase the rate of orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1988;94:278-284.
- Al-Hasani NR, Al-Bustani AI, Ghareeb MM, et al. Clinical efficacy of locally injected calcitriol in orthodontic tooth movement. Int J Pharm Pharm Sci. 2011;3(suppl 5):139-143.
- Yamasaki K, Miura F, Suda T. Prostaglandin as a mediator of bone resorption induced by experimental tooth movement in rats. J Dent Res. 1980;59:1635-1642.
- Chumbley AB, Tuncay OC. The effect of indomethacin (an aspirin-like drug) on the rate of orthodontic tooth movement. Am J Orthod. 1986;89:312-314.
- Mohammed AH, Tatakis DN, Dziak R. Leukotrienes in orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1989;95:231-237.
- Eells JT, Wong-Riley MT, VerHoeve J, et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4:559-567.
- Ying R, Liang HL, Whelan HT, et al. Pretreatment with near-infrared light via light-emitting diode provides added benefit against rotenone- and MPP+-induced neurotoxicity. Brain Res. 2008;1243:167-173.
- Coombe AR, Ho CT, Darendeliler MA, et al. The effects of low level laser irradiation on osteoblastic cells. Clin Orthod Res. 2004;4:3-14.
- Campanha BP, Gallina C, Geremia T, et al. Low-level laser therapy for implants without initial stability. Photomed Laser Surg. 2010;28:365-369.
- Khadra M, Lyngstadaas SP, Haanaes HR, et al. Effects of laser therapy on attachment, proliferation and differentiation of human osteoblast-like cells cultured on titanium implant material. Biomaterials. 2005;26:3,503-3,509.
- Karu TI, Pyatibrat LV, Kolyakov SF, et al. Absorption measurements of cell monolayers relevant to mechanisms of laser phototherapy: reduction or oxidation of cytochrome c oxidase under laser radiation at 632.8 nm. Photomed Laser Surg. 2008;26:593-599.
- Kim, SJ, Moon SU, Kang SG, et al. Effects of low-level laser therapy after Corticision on tooth movement and paradental remodeling. Lasers Surg Med. 2009;41:524-533.
- Loos, S, Shaughnessy, T, et al. Ongoing clinical research and evaluation. Canton and Suwanee, GA.
- Yoshida T, Yamaguchi M, Utsunomiya T, et al. Low-energy laser irradiation accelerates the velocity of tooth movement via stimulation of the alveolar bone remodeling. Orthod Craniofac Res. 2009;12:289-298.
- Yamaguchi M, Hayashi M, Fujita S, et al. Low-energy laser irradiation facilitates the velocity of tooth movement and the expressions of matrix metalloproteinase-9, cathepsin K, and alpha(v) beta(3) integrin in rats. Eur J Orthod. 2010;32:131-139.
- Altan BA, Sokucu O, Ozkut MM, et al. Metrical and histological investigation of the effects of low-level laser therapy on orthodontic tooth movement. Lasers Med Sci. 2012;27:131-140.
- Kawasaki K, Shimizu N. Effects of low-energy laser irradiation on bone remodeling during experimental tooth movement in rats. Lasers Surg Med. 2000;26:282-291.
- Baloul SS, Gerstenfeld LC, Morgan EF, et al. Mechanism of action and morphologic changes in the alveolar bone in response to selective alveolar decortication-facilitated tooth movement. Am J Orthod Dentofacial Orthop. 2011;139(suppl 4):S83-S101.
- Yu JY, Lee W, Park JH, et al. Histologic effects of intentional-socket-assisted orthodontic movement in rabbits. Korean J Orthod. 2012;42:207-217.
- Wilcko WM, Ferguson DJ, Bouquot JE, et al. Rapid orthodontic decrowding with alveolar augmentation: case report. World J Orthod. 2003;4:197-205.
- Wilcko MT, Wilcko WM, Pulver JJ, et al. Accelerated osteogenic orthodontics technique: a 1-stage surgically facilitated rapid orthodontic technique with alveolar augmentation. J Oral Maxillofac Surg. 2009;67:2149-2159.
- Wilcko WM, Wilcko T, Bouquot JE, et al. Rapid orthodontics with alveolar reshaping: two case reports of decrowding. Int J Periodontics Restorative Dent. 2001;21:9-19.
- Sharath K, Amitha R, Biju T, et al. Periodontally accelerated osteogenic orthodontics: review on a surgical technique and a case report. Journal of Interdisciplinary Dentistry. 2012;2:179-184.
- Einy S, Horwitz J, Aizenbud D. Wilckodontics—an alternative adult orthodontic treatment method: rationale and application. Alpha Omegan. 2011;104(3-4):102-111.
- Jofre J, Montenegro J, Arroyo R. Rapid orthodontics with flapless piezoelectric corticotomies: first clinical experiences. Int J Odontostomat. 2013;7:79-85.
- Keser EI, Dibart S. Sequential piezocision: a novel approach to accelerated orthodontic treatment. Am J Orthod Dentofacial Orthop. 2013;144:879-889.
- Uzuner FD, Darendeliler N. Dentoalveolar surgery techniques combined with orthodontic treatment: a literature review. Eur J Dent. 2013;7:257-265.
- Yu H, Jiao F, Wang B, et al. Piezoelectric decortication applied in periodontally accelerated osteogenic orthodontics. J Craniofac Surg. 2013;24:1750-1752.
- Grenga V, Bovi M. Corticotomy-enhanced intrusion of an overerupted molar using skeletal anchorage and ultrasonic surgery. J Clin Orthod. 2013;47:50-55.
- Mittal SK, Sharma R, Singla A. Piezocision assisted orthodontics: a new approach to accelerated orthodontic tooth movement. Journal of Innovative Dentistry. 2011;1. journal.pdmdentalcollege.com/issue1/reviewarticle/article5.html. Accessed on September 10, 2014.
- Teixeira CC, Khoo E, Tran J, et al. Cytokine expression and accelerated tooth movement. J Dent Res. 2010;89:1135-1141.
- Hoogeveen EJ, Jansma J, Ren Y. Surgically facilitated orthodontic treatment: a systematic review. Am J Orthod Dentofacial Orthop. 2014;145(suppl 4):S51-S64.
- Khoo E, Tran J, Raptis M, et al. Accelerated Orthodontic Treatment [research paper]. Presented at New York University Orthodontic Conference; New York, NY, November 2011.
- Alikhani M, Raptis M, Zoldan B, et al. Effect of micro-osteoperforations on the rate of tooth movement. Am J Orthod Dentofacial Orthop. 2013;144:639-648.
Dr. Gray is a 1986 graduate of Georgetown University School of Dentistry. He is also a graduate of the Pacific Aesthetic Continuum and the Pankey Continuum. He is a Master and Lifelong Learning and Service Recognition in the AGD and a Fellow in the International Congress of Oral Implantologists, International College of Dentists, and Academy of Dentistry International. The ADA appointed him as one of 2 practicing general practitioners (GPs) to the Council on Scientific Affairs. He is also an instructor at the L. D. Pankey and Dawson Institutes and 9 universities. He is the longest-tenured faculty member at Align Technology and has certified more than 19,300 GPs in the Invisalign process since 2001. He is an Invisalign Premier Provider and maintains a full-time, fee-for-service, private practice in Washington, DC. A number of dental manufacturers and nonprofit clinical research facilities rely on his input for product evaluation, research and development. He has been listed as one of Dentistry Today’s Leaders in Continuing Education since 2006 and has lectured on new dental technologies in more than 200 US cities and 10 countries since 1994. He can be reached at (202) 244-4111 or via e-mail at info@smiledc.com.
Disclosure: Dr. Gray is a faculty member at Aligntech Institute (Align Technologies).











