INTRODUCTION
With new emerging technology, ceramic implants are some of the most innovative products that provide an alternative to traditional titanium implants. They have been shown to be strong, aesthetic, and peri-implant-tissue friendly, as well as a metal-free option. Some of the modern ceramic implant systems, such as Zeramex XT (Dentalpoint AG), allow for a metal-free solution for long-term success. This offers a choice to those patients who have a titanium sensitivity or otherwise wish to use a nonmetal solution.1-3 In the cosmetic zone (maxillary anterior region), placing any type of implant can be challenging, especially with adjacent natural teeth. Zirconia offers an inherent white color to mask any shimmer or show-through, making a “gray” shadow a thing of the past. Thin biotype tissue is especially a characteristic that has always been an aesthetic risk and can be minimized with this innovative implant system and its natural tooth color. Zirconia has also been shown to provide optimal blood circulation around the implant body, thus increasing the long-term stability of the perio-implant complex.
The following clinical case demonstrates the use of the Zeramex 2-piece ceramic implant that uses a unique carbon fiber fixation screw to offer a true metal-free solution for the aesthetic zone.
CASE REPORT
A 54-year-old female presented to our office to learn about some options to replace failing partial RCT on her upper right maxillary central incisor (Figure 1). Tooth No. 8 was extracted using W&H piezo EXT 1 and EXT 2 tips to reduce the risk of a buccal fracture. The site was preserved with a cortico-cancellous allograft (Newport Surgical) and allowed to heal for 5 months (Figure 2). After healing, smile photos were taken to assess the overall aesthetic risk of the case. Treatment options were discussed with the patient. With a ridge width of less than 6.5 mm, the patient decided to use a ceramic implant to minimize the potential for gray show-through. We proposed doing a veneer graft as well as a connective tissue graft to minimize these risks if a titanium implant were placed. The patient declined these surgical interventions (Figures 3 and 4).
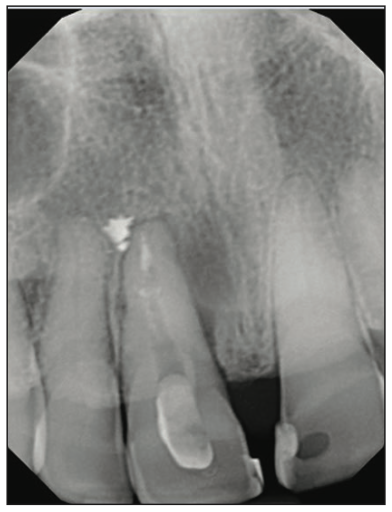
Figure 1. Preoperative situation: a failing RCT with poor prognosis for retreatment.
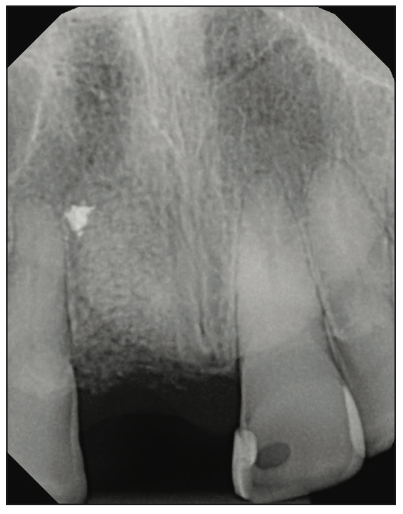
Figure 2. Post-extraction and healing after 5 months with cortico-cancellous bone (Newport Surgical).
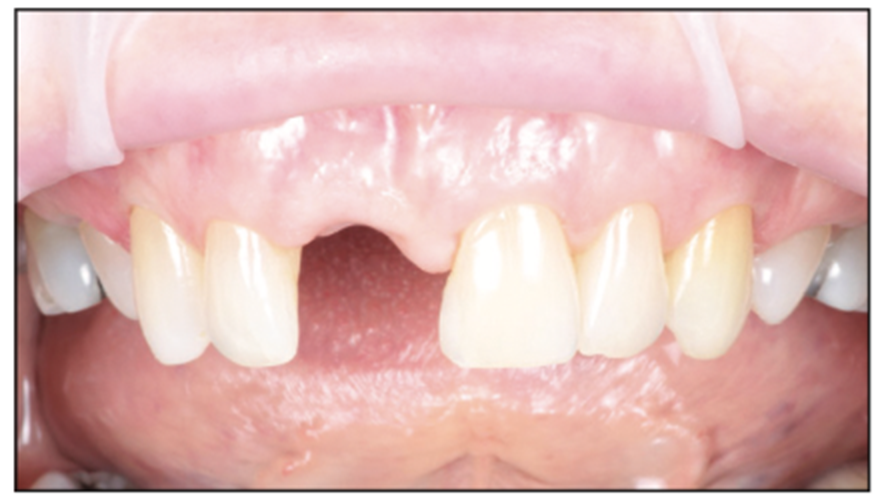
Figure 3. Healed site pre-op situation, buccal view.
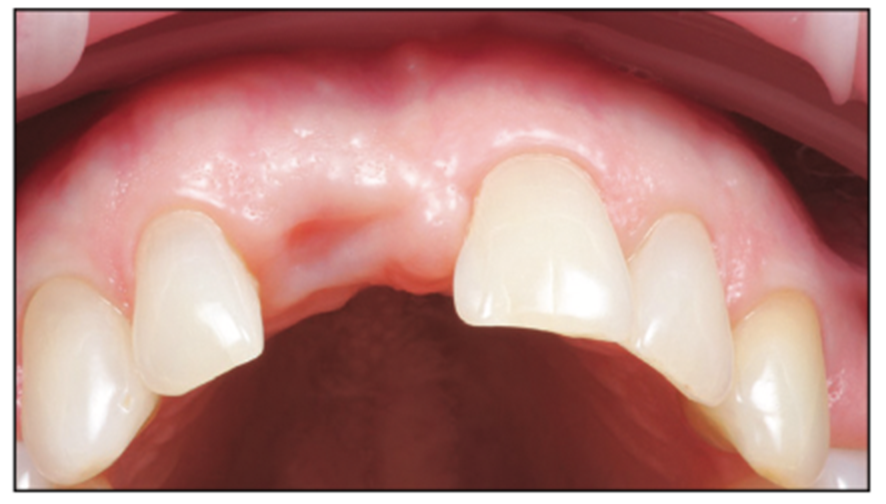
Figure 4. Healed site occlusal view.
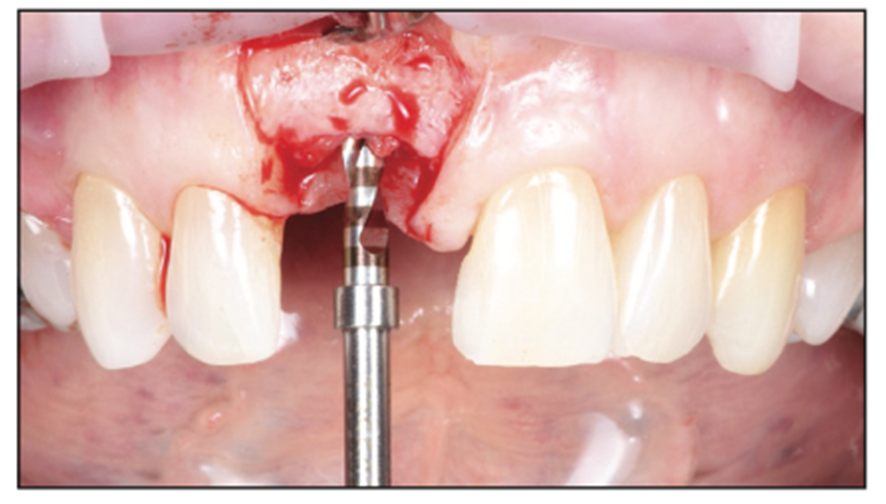
Figure 5. Papilla-sparing incision to visualize the reconstructed site and initial osteotomy to depth.
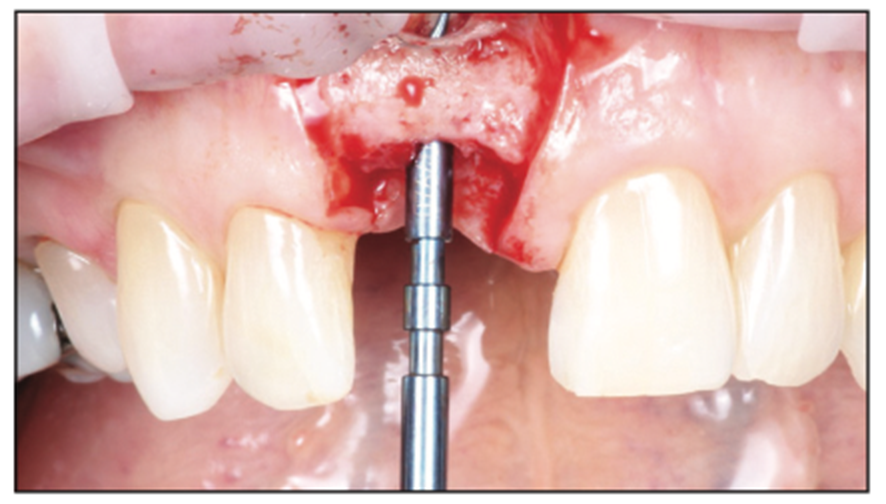
Figure 6. Depth gauge to visually and radiographically verify the planned implant position and depth.
A 2-part reversible screwable zirconium oxide implant (Zeramex XT) made of aluminum-toughened zirconia (ATZ) with a reduced diameter of 3.5 mm and a length of 12 mm was placed under local anesthesia with minimal flap formation. It was then closed with an end cap, and the tissue was repositioned and sutured (Figures 5 to 10).
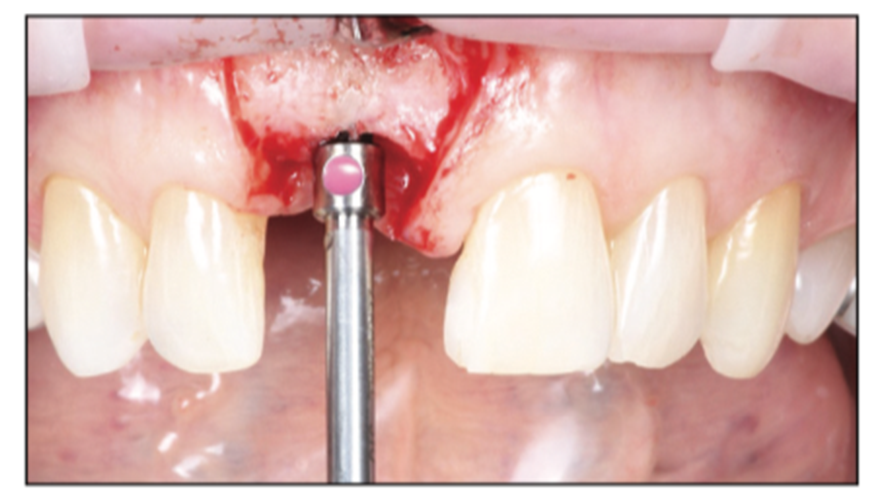
Figure 7. Narrow platform final shaping drill to depth.
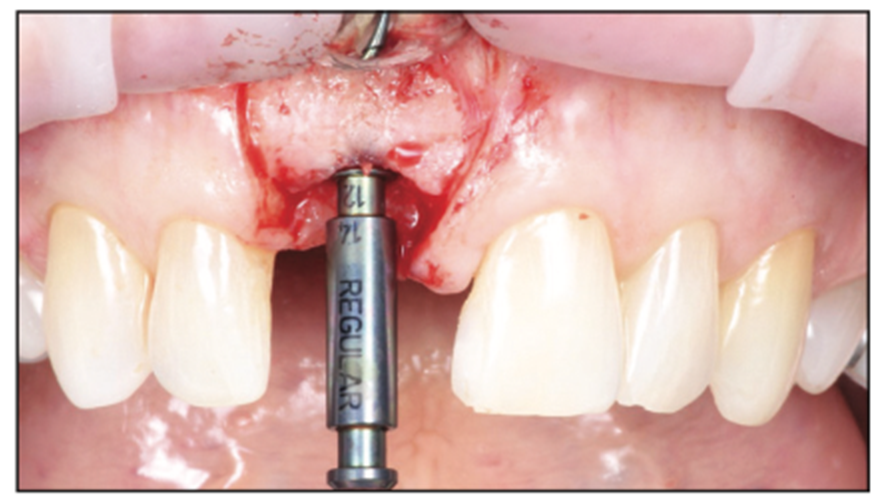
Figure 8. Regular platform final shaping drill to depth.
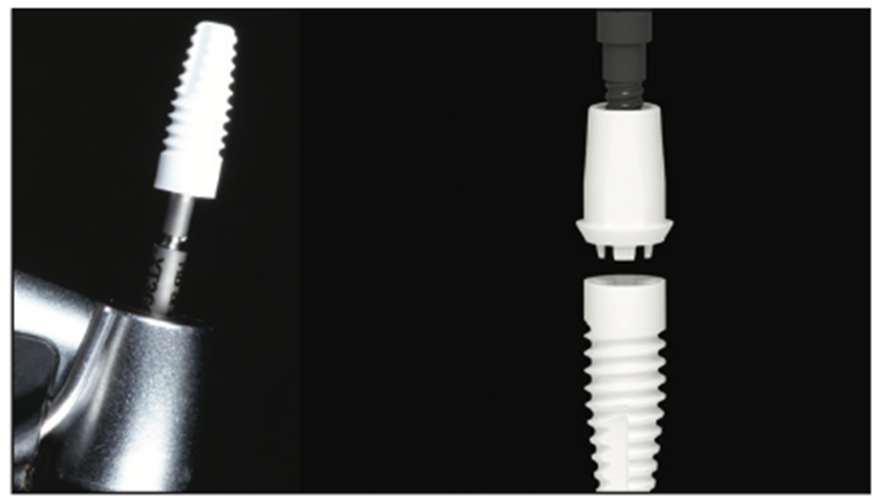
Figure 9. Zeramex 4.2- × 12-mm zirconia implant (2-piece) (Dentalpoint AG) with a carbon fiber final abutment screw.
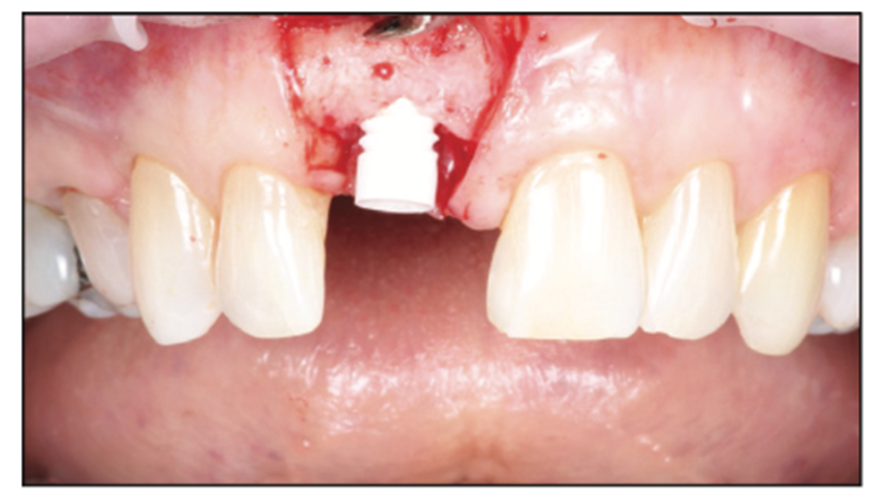
Figure 10. Implant delivery with a handpiece to 75% of depth. Final seating should be done with a torque wrench.
Surgical Phase
Following the suggested protocol from the manufacturer, the site was prepared for the 2-piece implant placement that was very similar to a titanium variant. It is important to note that the site must be prepared adequately to prevent excessive implant insertion torque and that the use of the drill tap is necessary. One important note when surgically placing the zirconia implant is that the implant is placed 1.6 mm to 0.6 mm supracrestally (Figures 11 to 16). The connection point is to be above the crest of bone. The implant is sandblasted and etched on the body to create a micro-roughened surface with a zone near the neck of the implant that is to not allow for true tissue adhesion.
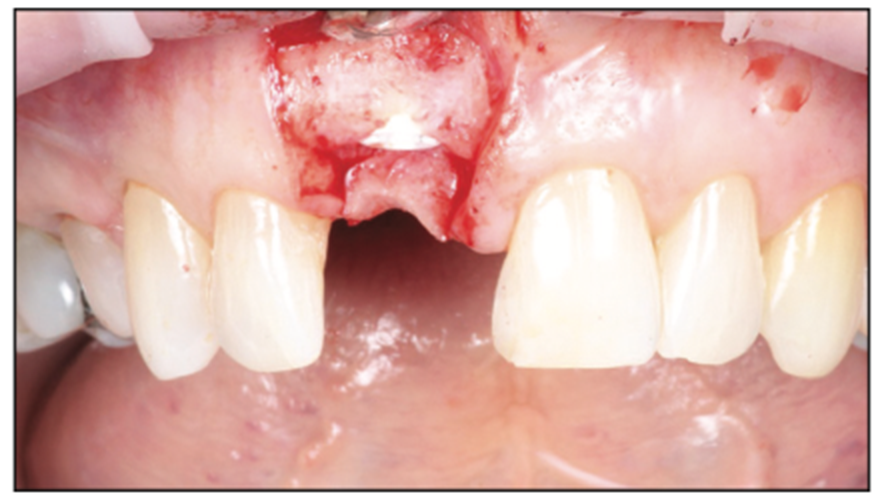
Figure 11. Buccal view of the final implant placement 0.6 mm supracrestal (can be anywhere from 1.6 to 0.6 mm supracrestal).
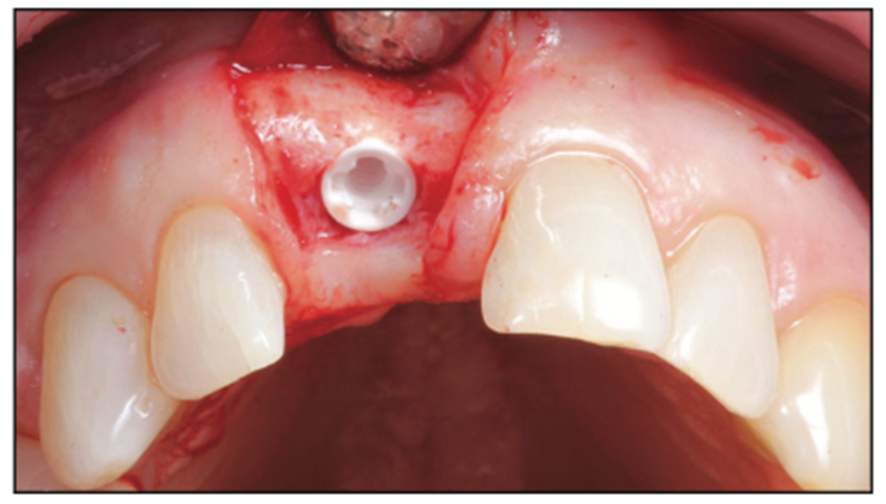
Figure 12. Occlusal view of the final implant placement.
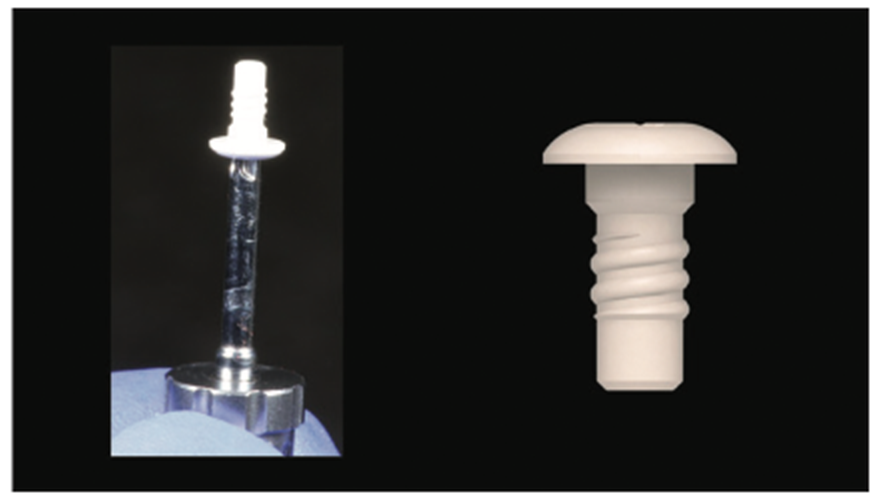
Figure 13. Flat cover screw (made of metal-free PEEK material).
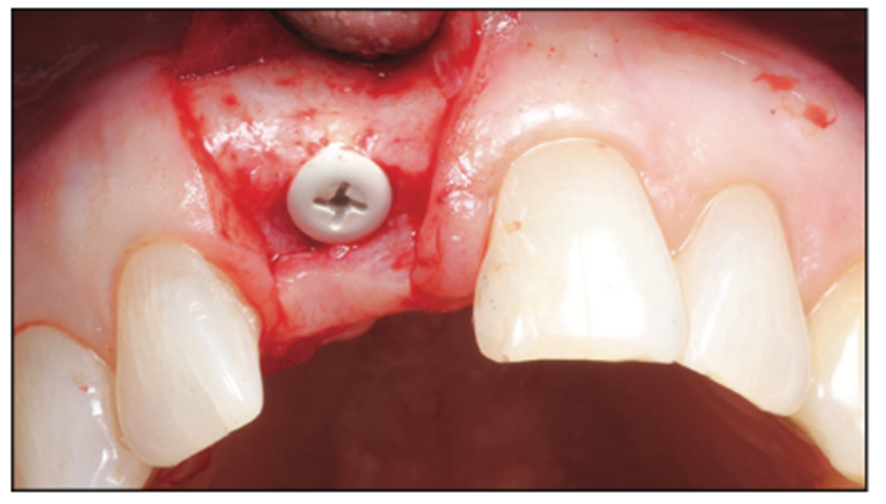
Figure 14. Flat cover screw installed into a Zeramex implant and hand-tightened.
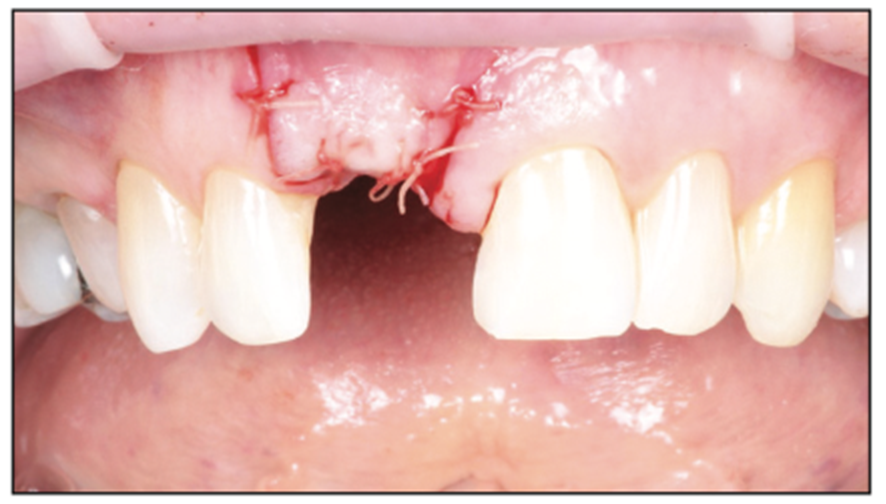
Figure 15. Closure with resorbable suture (Newport Surgical).
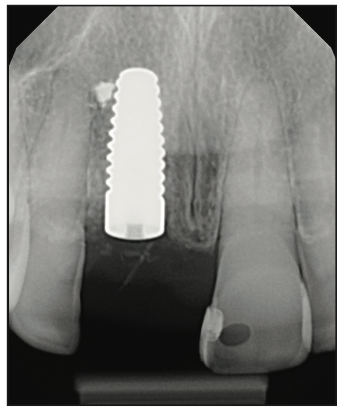
Figure 16. Final radiograph verification of Zeramex placement 1.5 mm away from adjacent tooth No. 7 and outside of the incisive canal.
Soft-Tissue Management and Final Prosthetic Restoration
After the healing phase was completed, the implant was exposed with a diode laser (Brasseler BLU [Brasseler USA]), and a peak Zeramex 3-mm-tall healing cap was placed (Figures 17 and 18). Healing was allowed to continue for 2 weeks (Figures 19 and 20).
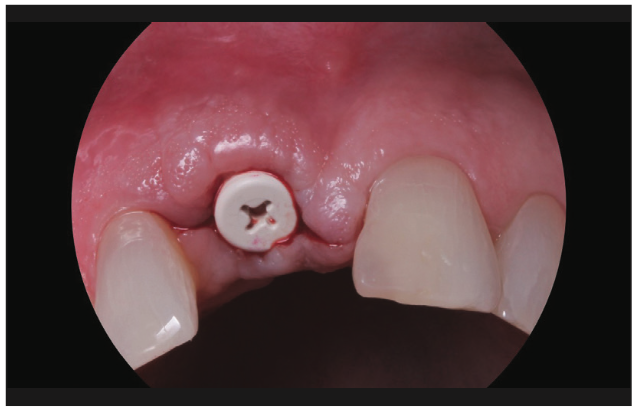
Figure 17. Uncovery of the Zeramex implant and tall healing cap placed (PEEK material).
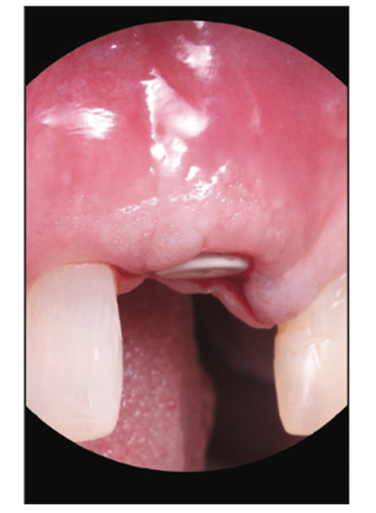
Figure 18. Uncovery of the Zeramex implant (buccal view) to show robust tissue health with no gray shimmer or show-through of the material.
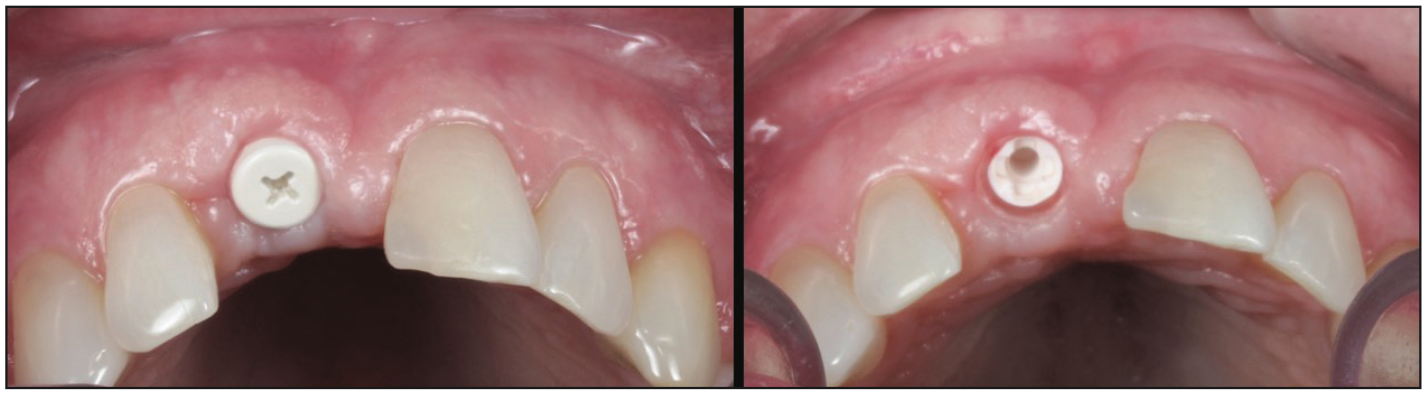
Figure 19. Two-week heal check to demonstrate tissue health (occlusal view).
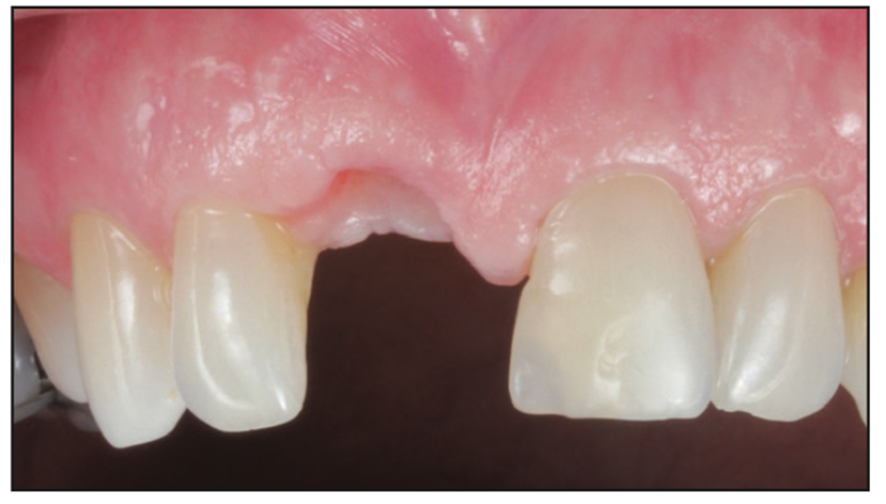
Figure 20. Two-week heal check, buccal view.
After the soft-tissue healing phase, the gingival profile was evaluated, showed no signs of inflammation, and demonstrated excellent biocompatibility with these materials. A standard restorative process of using a closed tray impression post was planned. After verification with an intraoral PA, a PVS impression was taken (Capture [Glidewell]) (Figure 21). This was sent to the lab to fabricate an all-zirconia crown (BruxZir [Glidewell]) (Figure 22).

Figure 21. Closed tray Zeramex impression post for a traditional restorative workflow.
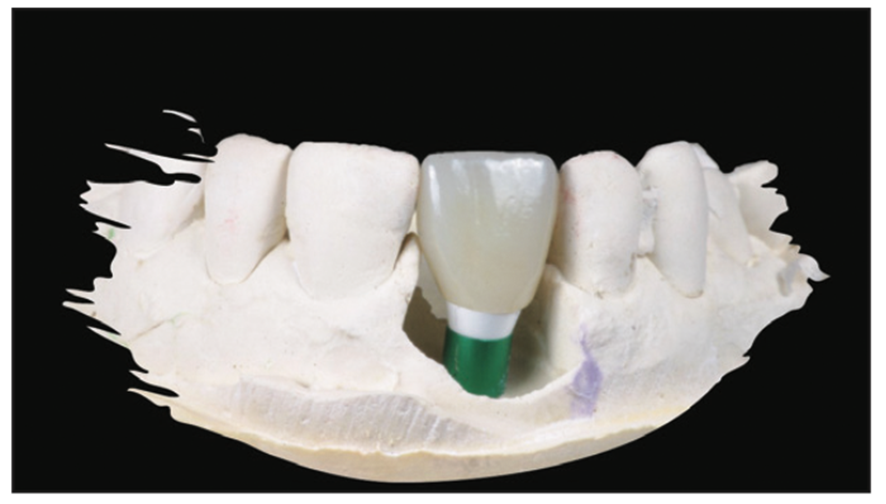
Figure 22. Final BruxZir crown (Glidewell) over a customized stock abutment.
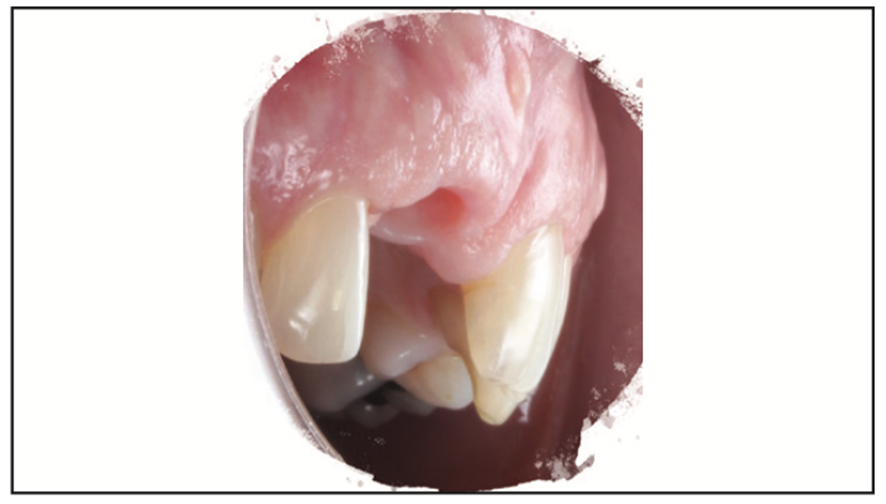
Figure 23. Tissue profile on the day of delivery of the customized zirconia abutment and BruxZir crown.
A Zeramex standard abutment also made of ATZ was fixed in the implant with a Vicarbo screw (Zeramex XT). This screw made of carbon fiber longitudinal strands, molded slightly larger than the internal aspect of the implant, allows transmission of the forces of mastication to be absorbed while providing a tight, hermetically sealed connection. The carbon fiber screw affords this complex with almost 2 times the tensile strength of a titanium fixation screw (Figure 23).
The final BruxZir crown was tried in and cemented with Infinity cement (DenMat) (Figures 24 and 25).
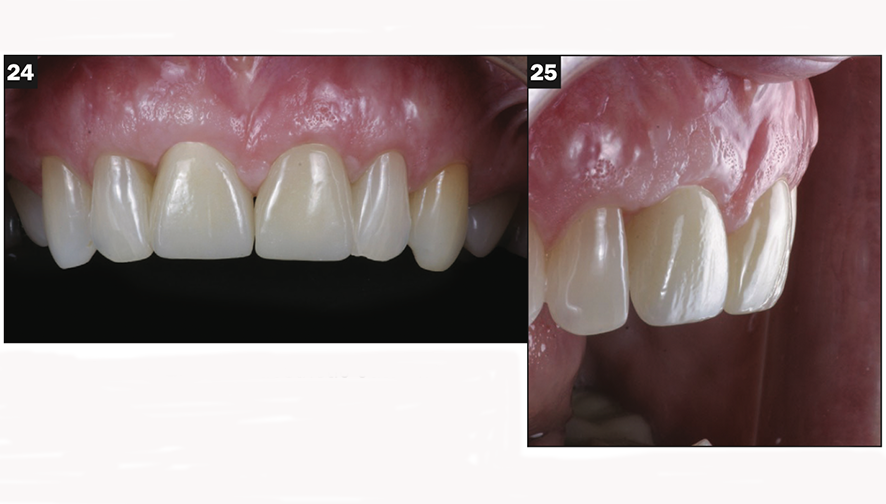
Figures 24 and 25. Final aesthetic outcome.
DISCUSSION
As the number of titanium-based implants being placed per year continues to rise, so does the clinically observed incidence of peri-implantitis. With the osseointegration rate of ceramic implants closely approximating that of titanium, an alternative exists that can offer a viable solution to reduce the amount of biofilm around the fixture as well as the peri-implant soft tissue.
With the inherent gray color of titanium-based fixtures, color matching and blending into the soft and hard tissues will remain a challenge. This has been minimized with the use of ceramic abutments; however, this does not always reduce the metal darkening of the peri-implant soft tissue. Ceramic implants, with their white color, offer optical properties that allow for more natural, lifelike aesthetic results similar to that of the natural tooth.
CONCLUSION
In conclusion, zirconia implants now have overcome the limitations of previous generations by being milled from ATZ to offer high strength. A strong, metal-free connection has been designed to minimize bacteriological colonization and deposition on the surface of the abutment. The naturally white color allows for no dark shadows, no shimmer, and optimal blood circulation that is more similar to a natural tooth, thus promoting inflammation-free gingival tissue.
These are very positive clinical results, but long-term studies are necessary to further verify the effectiveness and success rates of zirconia implants. With a remarkably similar surgical and prosthetic protocol, most clinicians can transition to offer another choice with a minimal learning curve. This treatment offered the patient the ability to have a natural aesthetic result with minimal additional procedures while maximizing gingival health and bone health.
REFERENCES
1. Möller B, Terheyden H, Açil Y, et al. A comparison of biocompatibility and osseointegration of ceramic and titanium implants: an in vivo and in vitro study. Int J Oral Maxillofac Surg. 2012;41(5):638-645
2. Wachi T, Shuto T, Shinohara Y, et al. Release of titanium ions from an implant surface and their effect on cytokine production related to alveolar bone resorption. Toxicology. 2015 Jan 2;327:1-9. Epub 2014 Nov 3. doi: 10.1016/j.tox.2014.10.016
3. Addison O, Davenport AJ, Newport RJ, et al. Do ‘passive’ medical titanium surfaces deteriorate in service in the absence of wear? J R Soc Interface. 2012 Nov 7;9(76):3161-4. Epub 2012 Jul 25.
ABOUT THE AUTHOR
Dr. Patel is a graduate of the University of North Carolina at Chapel Hill School of Dentistry and the Medical College of Georgia/American Academy of Implant Dentistry (AAID) MaxiCourse. He is a clinical instructor at the Reconstructive Dentistry Institute. Dr. Patel has placed more than 5,000 implants, published numerous articles in leading dental journals, and worked as a lecturer and clinical consultant on dental implants and prosthetics for various companies. He belongs to numerous dental organizations, including the ADA, the North Carolina Dental Society, and the AAID. He can be reached via email at pareshpateldds2@gmail.com or at the website implantsbyparesh.com.
Disclosure: Dr. Patel reports no disclosures.












