Candida albicans, a type of yeast, takes advantage of an enzyme produced by Streptococcus mutans to form a particularly intractable biofilm that can lead to early childhood caries. Now, researchers at the University of Pennsylvania School of Dental Medicine have pinpointed the surface molecules on the fungus that interact with the bacterially derived protein. Blocking that interaction impairs the yeast’s ability to form a biofilm with S mutans on the tooth surface.
“Instead of just targeting bacteria to treat early childhood caries, we may also want to target the fungi,” said senior author Hyun (Michel) Koo, DDS, MS, PhD, professor in the Department of Orthodontics and Divisions of Pediatric Dentistry and Community Oral Health. “Our data provide hints that you might be able to target the enzyme or cell wall of the fungi to disrupt the plaque biofilm formation.”
Candida can’t effectively form plaque biofilms on teeth on its own, nor can it bind S mutans, unless it’s in the presence of sugar. Children who consume sugary foods and beverages in excess are at risk for early childhood caries. The researchers previously discovered that the GftB enzyme, secreted by S mutans, uses sugar from the diet to manufacture glue-like polymers called glucans. Candida promotes this process, resulting in a sticky biofilm that lets the yeast adhere to teeth and bind to S mutans.
The researchers suspected that the outer portion of the Candida cell wall, comprising molecules called mannans, might be involved in binding GftB. So, they measured the binding strength between various mutant Candida strains and GtfB using biophysical methods. They found that the enzyme bound much more weakly to mutants that lacked components of the mannan layer than the wild-type Candida.
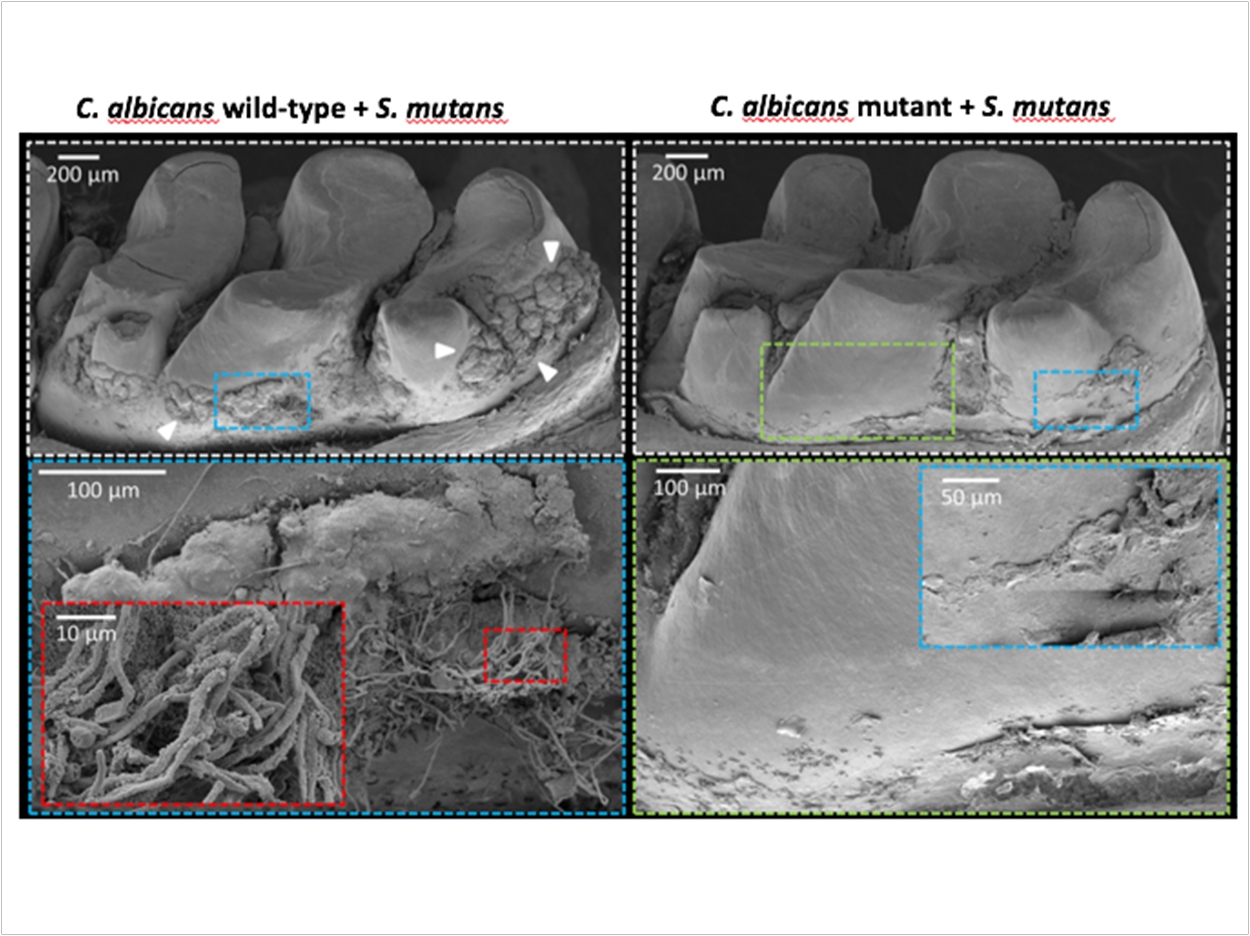
Next, the researchers examined the abilities of the mutant Candida to form biofilms with S mutans in a laboratory assay. The mutants that had impaired binding with GftB were mostly unable to form biofilms with S mutans, resulting in significantly fewer Candida cells and reduced production of the sticky glucans molecules.
Additionally, the researchers tested how stable the biofilms were when attached to a tooth-like surface. While low-shear stress, roughly equivalent to the force generated by taking a drink of water, removed only a quarter of the wild-type biofilm, the same force removed 70% of the biofilms with mutant Candida. When the forces were equivalent to a vigorous mouthrinse, the mutant biofilms were almost completely dislodged.
To ensure their findings translated to in vivo conditions, the researchers examined biofilm formation in a rodent model that can mimic the development of early childhood caries. When animals were infected with both S mutans and either of the wild type of defective mutant yeast strains, the researchers observed clear differences. While biofilm formation was abundant if the wild-type yeast was used, it was substantially reduced in animals infected with the mutant strain. More precise analysis revealed that these defective biofilms lacked viable Candida cells, and S mutans were reduced by more than fivefold.
According to the researchers, these findings point to a new direction for treatment of early childhood caries. The current standard of care, beyond the use of fluoride as a preventive approach, is to target only the bacteria with antimicrobials or to use surgical interventions if the tooth decay has become too severe. The researchers now are working on therapeutic approaches for targeted interventions with potential for clinical use.
“The disease affects 23% of children in the United States and even more worldwide,” said Koo. “In addition to fluoride, we desperately need an agent that can target the disease-causing biofilms and, in this case, not only the bacterial component but also the Candida.”
The study, “Candida Albicans Mannans Mediate Streptococcus Mutans Exoenzyme GtfB Binding to Modulate Cross-Kingdom Biofilm Development In Vivo,” was published by PLOS One.
Related Articles
Cleansing Crystals Kill Candida on Oral Appliances in Just 20 Minutes
Antimicrobial Protein Combats Oral Thrush
Stigmergy May Explain Biofilm Growth—And Bacterial Consciousness


