Botulism toxin (BTX) is produced during the growth and autolysis of the strictly anaerobic, spore-forming, Grampositive rod Clostridium botulinum. BTX can be differentiated serologically into 8 types. Recently, botulinum toxin type A (BTX A) has been recognized as an agent that can be used in the treatment of focal dystonias, including blepharospasm, oromandibular dystonias, and spasmodic torticollis.
BTX A acts as a presynaptic neurotoxin that blocks neuromuscular transmission by binding to receptor sites on motor or sympathetic nerve terminals, entering the nerve terminals, and inhibiting the release of acetylcholine. This inhibition occurs as the neurotoxin cleaves SNAP-25, a protein integral to the successful docking and release of acetylcholine from vesicles located within nerve endings. When BTX A is injected intramuscularly in therapeutic doses, it produces partial chemical denervation of the muscle, resulting in a localized reduction in muscle activity. This causes dose-dependent weakness or paralysis in skeletal muscle. The effect of BTX A can last from 3 to 6 months, and local paralysis is reversed chiefly by neural sprouting with reinnervation of the muscle. In addition, when injected intradermally, BTX A produces temporary chemical denervation of the sweat glands, resulting in local reduction of sweating.
The Food and Drug Administration (FDA) first approved BTX for use in focal dystonia in 1989. Following completion of clinical trials, the FDA approved BTX A use in treating cervical dystonias, primary axillary hyperhidrosis, blepharospasm, and strabismus. These clinical problems have been challenging to clinicians and generally treated surgically with poor results. BTX has been widely adapted for these applications because it provides a minimally invasive approach to treating these challenging clinical problems. One of the most popular and successful applications of BTX has been in the treatment of hyperkinetic facial lines.
BTX A is produced by Allergan and is supplied in 100-unit vials. One unit of BTX corresponds to the calculated median intraperitoneal lethal dose (LD50) in mice. Unopened BTX must be stored in a refrigerator (2º to 8ºC), and preservative-free normal saline is used for reconstitution. In general, 1 to 8 mL of saline is added to 1 vial, producing a concentration of 10 to 1.25 units per 0.1 mL. Once reconstituted, the effectiveness of BTX begins to diminish after 4 hours. Therefore, immediate administration of BTX is recommended. The recommended doses range from 5 units to 25 units per muscle. The cumulative dose of BTX treatment in a 30-day period should not exceed 200 units.1
TEMPOROMANDIBULAR DISORDERS (TMD)
Temporomandibular disorders (TMD) are a broadly defined group of nonodontogenic facial pain/disorders associated with the temporomandibular joint (TMJ) and/or associated muscles. Often, proper diagnosis of TMD problems can be challenging to clinicians as a result of the overlap of symptoms with other oral/dental/craniofacial disorders. Usual TMD symptoms can include but are not necessarily limited to the following: jaw pain, difficulty with jaw opening, earaches, headaches, pain behind the eyes, jaw joint popping and clicking, dizziness, and difficulty chewing food or occluding the teeth.
In recent years, there have been reports describing the use of BTX for several subcategories of TMD: bruxism, clenching, masseteric hypertrophy, recurrent dislocation of the temporomandibular joint, oromandibular dystonias, and chronic myogenous orofacial pain.2-12 Although BTX is not a panacea for the listed problems, this drug appears to be an important treatment option for many patients. This article will review the clinical problems for which BTX has been used, and the indications and reported effectiveness will be discussed.
TMJ Dislocation
Recurrent TMJ dislocation may be the result of one of several underlying problems. Often it is the result of a steep articular eminence and ligamental laxity. Patients with such an anatomic variance will often report that after yawning, the mandible cannot be closed. In some cases, these events resolve spontaneously, and in other situations a visit to the emergency room is necessary. With conservative therapy and restricted jaw-opening exercises, many patients can fully control TMJ dislocation. In a small percent of patients, the dislocation recurs, especially among elderly patients with neurologic disorders or tardive dyskinesia secondary to medications (eg, antidepressants).
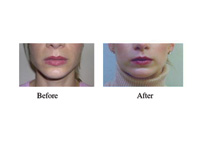 |
| Figure. Masseteric hypertrophy and myalgia successfully treated with BTX A injection. |
In the past, limited use of intermaxillary fixation or invasive surgery was used to treat recurrent dislocation of the TMJ. Currently, with electromyography-guided delivery of BTX A to the lateral pterygoid muscles, extreme range of motion of the mandible may be prevented for 3 to 4 months. Several case reports have been published that support the use of BTX A for recurrent dislocation of the TMJ.2-4
Masseteric Hypertrophy
Masseteric hypertrophy may be unilateral or bilateral. It is often associated with excessive chewing of gum or food and clenching habits while awake or asleep. It presents as a unilateral condition when the habit favors asymmetric use of the jaw. It may be associated with myogenous facial pain (usually the masseteric or temporalis) or may present alone as a cosmetic complaint. Several studies have shown that the use of BTX A at the masseteric bulge can result in significant reduction of the muscular mass, which results in a more ovoid appearance of the lower face. Furthermore, the reduction in muscle mass is accompanied by less myogenic pain and discomfort. Again, these effects usually last 3 to 4 months. However, with repeated treatments some patients do not require additional treatment due to a satisfactory reduction in masseteric muscle bulge and a reduced clenching habit. An adverse side effect of facial muscle weakness has been reported in some patients. This is most likely due to diffusion of BTX A to superficial muscles during masseteric injection.5-6
Chronic Myogenous Facial Pain/Myofacial Pain
Orofacial pain of muscular origin is often referred to as myofacial pain. The current conservative treatment approach includes the use of nonsteroidal anti-inflammatory medications, soft diet, and occlusal splint. Other medications such as muscle relaxants and tricyclic antidepressants have been used. Some clinicians advocate the use of other modalities such as massage therapy, ultrasound therapy, and transcutaneous electric nerve stimulation. More than 80% of patients respond to conservative therapy.13
For those patients who are refractory to these treatments and continue to suffer significant pain and dysfunction, treatment with BTX has been proposed. As mentioned previously, patients with masseteric hypertrophy and pain often respond well to BTX A administration to the affected muscles. In a randomized, blinded, placebo-controlled study by Von Linden, et al7, 90 patients with chronic facial pain were treated (60 BTX A, 30 placebo). Patients received on average 35 units of BTX A to affected muscles (masseter, temporalis, or medial pterygtoid), and 0.9% normal saline was used as a control. The data indicated that 91% of those who received BTX A reported improvement, with a significant mean reduction of approximately 3.2 on the visual analog pain scale (VAS 0 to 10).
It is believed that BTX A hinders trigeminal nerve activity not only by preventing the release of acetylcholine but also preventing the release of substance P. Substance P is a potent neurotransmitter that plays a role during neurological inflammation.7 Furthermore, BTX A therapy can indirectly alleviate pain of arthrogenic origin. This is achieved with the prolonged “joint-sparing” effect of diminished loading secondary to the decreased ability of the musculature to affect joint loading.8
A study by Freund, et al9 also supports the effectiveness of BTX A in the reduction of myogenic pain associated with the TMJ. Forty-six patients with TMD were treated with a total dose of 150 units of BTX A (50 units per masseter and 25 units per temporalis). During the following 8 weeks these patients had a mean 3-point reduction in the 0 to 10 VAS pain score. There were no controls in this study.9
A study by Nixdorf, et al10 of 15 patients (10 completed the study) examined the use of BTX A for chronic myogenous orofacial pain. This report did not show a statistically significant improvement in the group that received BTX A versus normal saline. In the discussion, they acknowledged the small number of patients in the study.10
Bruxism and Clenching
Bruxism is a diurnal or nocturnal parafunctional habit of the jaws that includes clenching or grinding of teeth. Bruxism is thought to be a dystonia, although its origin in the central nervous system is not understood.11 Severe bruxism has been reported in anoxic brain injury and in patients with neurological disorders such as Rett syndrome. Certain medications can cause tardive dyskinesia and bruxism habits. Other etiologies for bruxism are head trauma, Parkinsonism, and neurodegenerative disorders.
A study by Tan and Jankovic11 successfully treated severe bruxism with BTX A. Approximately 65 units were injected per masseteric muscle in patients with long-standing (14±10 years) bruxism habits. The mean duration of response was 19 weeks, and the mean peak effect on a scale of 0 to 4 (in which 4 equals to total abolishment of grinding) was 3.4± 0.9. Only one subject reported having dysphagia following injection of BTX A.11
Oromandibular Dystonia
Oromandibular dystonia (OMD) refers to involuntary spasms of masticatory, lingual, and pharyngeal muscles resulting in tongue protrusion, persistent jaw opening and closing, or jaw deviation. OMD can be idiopathic or can be caused by a wide range of neurological disorders. A study by Tan and Jankovic12 examined the effect of BTX A on OMD; 202 patients were treated with injection of the masseter and submentalis complex. On a zero to 4 scale, with 4 being complete abolition of dystonia, the authors reported a mean reduction of 3.1. The best results were seen in patients with jaw-closing oromandibular dystonia. This report did observe that 31.5% of patients had adverse side effects, which included dysarthria and dysphagia. The high incidence of adverse side effects may be due to BTX A overdosing.
DISCUSSION
There is a growing body of evidence that supports the use of BTX A as a treatment modality for various TMD. Several subcategories of TMD including bruxism, clenching, masseteric hypertrophy, recurrent dislocation of the TMJ, oromandibular dystonias, and chronic myogenous orofacial pain have been effectively treated with BTX. However, without FDA approval, using BTX for treatment of TMD (with the exception of oromandibular dystonia) is an off-label application.
BTX has become a treatment modality in many healthcare disciplines. Its effects are reversible, and administration is minimally invasive. Adverse side effects such as dysarthria and dysphagia are observed when overdosing occurs or the injection misses the target muscle and the medication diffuses into adjacent structures. In addition, this treatment can be expensive ($474 for a 100-unit vial; adding a professional fee can bring the cost to more than $1,100 for a therapy that lasts up to 4 months). For off-label application, insurance does not cover BTX injections. Injection of BTX A should be performed by a clinician with knowledge of its pharmacology and the relevant anatomy of the sites receiving the injection. Finally, Allergan offers a comprehensive manufacturer’s package insert, which includes FDA-approved use of the medication as well as contraindications for its use, such as hypersensitivity, pre-existing neuromuscular disorders (eg, muscular dystrophy), and dysphagia. Although there has been no fatal hypersensitivity/allergic reaction to BTX, severe dysphagia, which resulted in aspiration pneumonia and death, has been reported.1
References
1. Botox [package insert]. Irvine, Calif: Allergan Inc; 2004.
2. Daelen B, Thorwirth V, Koch A. Treatment of recurrent dislocation of the temporomandibular joint with type A botulism toxin. Int J Oral Maxillofac Surg. 1997;26:458-460.
3. Aquilina P, Vickers R, McKellar G. Reduction of a chronic bilateral temporomandibular joint dislocation with intermaxillary fixation and botulinum toxin A. Br J Oral Maxillofac Surg. 2004;42:272-273.
4. Moore AP, Wood GD. Medical treatment of recurrent temporomandibular joint dislocation using botulinum toxin A. Br Dent J. 1997;183:415-417.
5. Bentsianov B, Francis A, Blitzer A. Botulinum toxin treatment of temporomandibular disorders, masseteric hypertrophy, and cosmetic masseter reduction. Oper Tech Otolaryngol Head Neck Surg. 2004;15(2):110-113.
6. Park MY, Ahn KY, Jung DS. Botulism toxin type A treatment for contouring of the lower face. Dermatol Surg. 2003;29:477-483.
7. von Lindern JJ, Niederhagen B, Berge S, et al. Type A botulinum toxin in the treatment of chronic facial pain associated with masticatory hyperactivity. J Oral Maxillofac Surg. 2003;61:774-778.
8. Schwartz M, Freund B. Treatment of temporomandibular disorders with botulinum toxin. Clin J Pain. 2002;18(6 suppl):S198-S203.
9. Freund B, Schwartz M, Symington JM. Botulinum toxin: new treatment for temporomandibular disorders. Br J Oral Maxillofac Surg. 2000;38:466-471.
10. Nixdorf DR, Heo G, Major PW. Randomized controlled trial of botulinum toxin A for chronic myogenous orofacial pain. Pain. 2002;99:465-473.
11. Tan EK, Jankovic J. Treating severe bruxism with botulinum toxin. J Am Dent Assoc. 2000;131:211-216.
12. Tan E, Jankovic J. Botulinum toxin A in patients with oromandibular dystonia: long-term follow-up. Neurology. 1999;53:2102-2107.
13. Peterson LJ, ed. Principles of Oral and Maxillofacial Surgery. 2nd ed. Hamilton, Ontario, Can: B.C. Decker; 2004:chap 48.
Dr. Chang completed her dental degree at Columbia University School of Dental and Oral Surgery and her medical degree at the University of Connecticut Health Center. She completed her residency in oral and maxillofacial surgery in June of 2004. At the 2004 annual American Association of Oral and Maxillofacial Surgeons meeting, she presented her work, “Analysis of Inflammatory Mediators in TMJ Synovial Fluid Lavage Samples of Symptomatic Patients and Asymptomatic Controls,” which won the best abstract award for the session. In May of 2005 she joined Columbia University as a full-time faculty member in the division of oral and maxillofacial surgery. She can be reached at (212) 305-7626 or hkc3@columbia.edu.



