Full-Arch Removable Treatment
INTRODUCTION
In part 1 of this series, in the March 2023 issue, we discussed single and multi-unit restorations. This is part 2 of the series, which will focus on implant-retained and -supported full-arch removable treatment with ceramic dental implants.
Background
As the baby boomer population is entering into the senior phase of their lives, there are a large number of edentulous adults in the United States seeking treatment. According to the American College of Prosthodontics and the National Center for Health Statistics,1 there are 36 million completely edentulous adults in the United States. With implant restorations being very common nowadays and highly successful, this population cohort is aware of and requesting implant treatment on a daily basis. In recent years, a unique phenomenon has occurred where patients are requesting less invasive procedures and materials. In the case of dental implants, the request for ceramic implants from patients has been on a rapid rise. Implant treatment modalities for complete edentulism can be divided into 2 categories: fixed and removable. The fixed option tends to be more appealing due to its similarities with native dentition; however, cost, stringent hygiene, and maintenance requirements become an obstacle for some patients.2 The removable overdenture option is preferred in cases of financial limitations, oral hygiene limitations, and/or limited availability of bone. In addition to this, the removable option is more attractive when aesthetics are of concern, especially if the transition line cannot be hidden or additional lip support is required.3 The survival rates of implants supporting maxillary overdentures in one long-term study was shown to be 91.9% vs 98.6% in the mandible.4 Age, sex, and splinting do not influence survival rates.4,5 A minimum number of 4 implants is recommended in the maxilla for the support of a maxillary overdenture.6,7
There is very little literature on ceramic implants and overdentures. The authors were unable to find any research on 2-piece ceramic implants used for overdentures. The reason for this is twofold: First, ceramic implants have a shorter history of use in comparison to titanium implants. Second, the locator components for ceramic implants have been introduced recently and are only commercially available with a few ceramic implant systems, including PUREloc (Straumann) (Figure 1) and Docklocs (Zeramex) (Figure 2). With the advancement in ceramic implant manufacturing, composites of ceramics have emerged as reliable implant materials, thereby expanding the applications and prosthetic range of ceramic implants. The zirconia composites yttria-stabilized zirconia, zirconia-toughened alumina, and alumina-toughened zirconia have primarily emerged as the 3 forms of zirconia used in the manufacturing of load-bearing ceramics.8 Yttria has been used to increase the structural stability of zirconia by limiting aging and improving its optical properties and aesthetic value.9 Furthermore, there are 2 major methods of manufacturing ceramic implants: milling of a zirconia bloc or injection molding of a ceramic slurry. Implants produced by both methods have been tested for structural and mechanical stability, and no statistically significant difference was observed in their responses to artificial aging and mechanical stress tests.10 In light of these technical and manufacturing improvements, the range of ceramic implant applications is expanding, and more prosthetic components are becoming available. This allows the clinician and the lab to offer more metal-free solutions to address patients’ edentulism. Osman, Payne, et al11 used one-piece ceramic implants in their pilot study with ball attachments for maxillary and mandibular overdentures. Out of 28 implants, 4 failed to integrate prior to loading and were replaced. At one-year followup, all remaining implants survived and were successful. In another study, Osman, Swain, et al12 compared one-piece titanium implants to one-piece ceramic implants, both with ball-type attachments, and the success rates after one year in the maxilla were significantly low (71.9% for titanium and 55% for ceramic). Part of the reason for these low success rates can be attributed to the small sample size as well as the fracture of 2 ceramic implants in the maxilla. The implant literature has demonstrated a slightly higher failure rate of dental implants in the maxilla. It has been 10 years since that study, and thanks to material engineering and advanced manufacturing protocols, there have been significant changes and improvements in load-bearing ceramic implant material toughness, flexural strength, and overall composition. To the best of the authors’ knowledge, no studies are available on 2-piece ceramic implants with locator-type abutments. This is an area where more research is needed to validate the long-term success and indications for removable full-arch solutions on ceramic implants.
CASE REPORT
Complete Maxillary Edentulism
An 86-year-old female patient presented to our clinic for a tooth replacement consultation. She had been fully edentulous in the maxilla for the past decade and was not happy with her complete dentures. Her main complaints were dietary restrictions and the inability to taste her food since her palate was covered. The patient initially resisted implants because she did not want to have metals inside her body. She heard about ceramic dental implants and requested them as a solution due to lifestyle preferences.
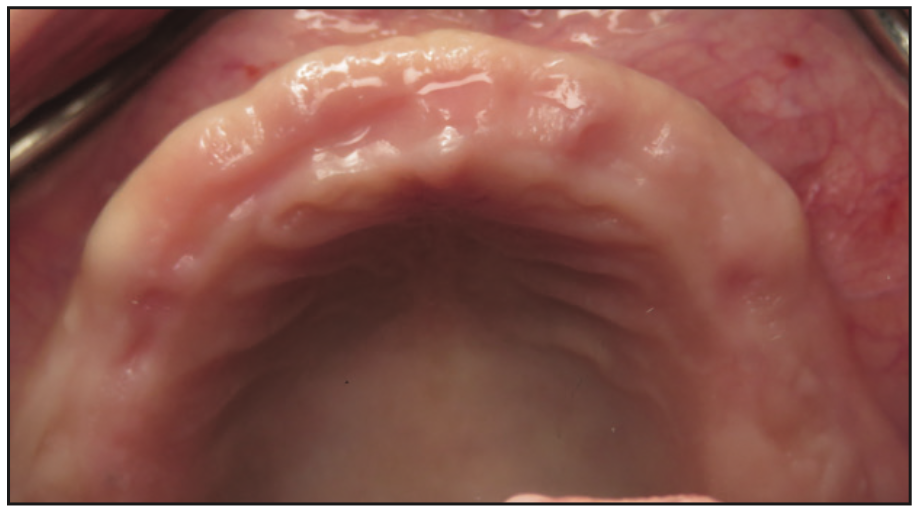
Figure 3. Preoperative photo of the edentulous ridge, showing adequate bone volume and keratinized tissues.
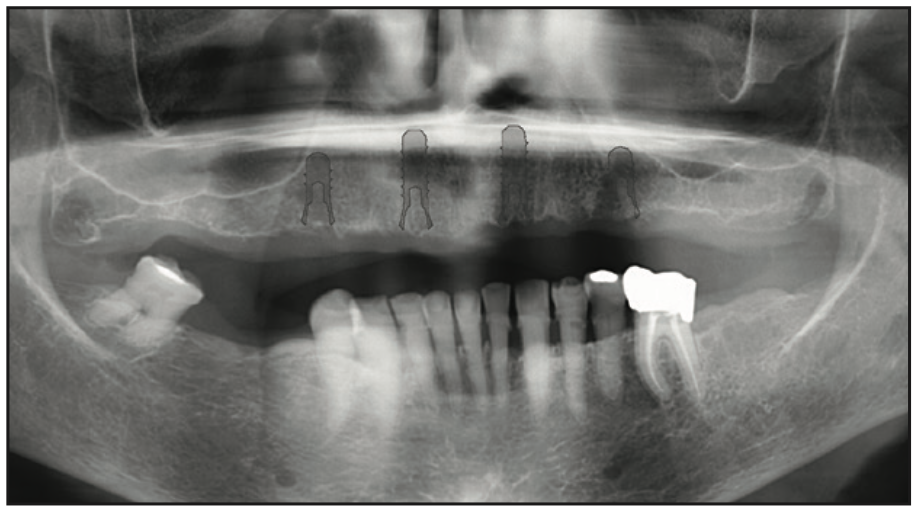
Figure 4. Pre-op panoramic radiograph used to identify vital structures and virtually plan the implant placement.
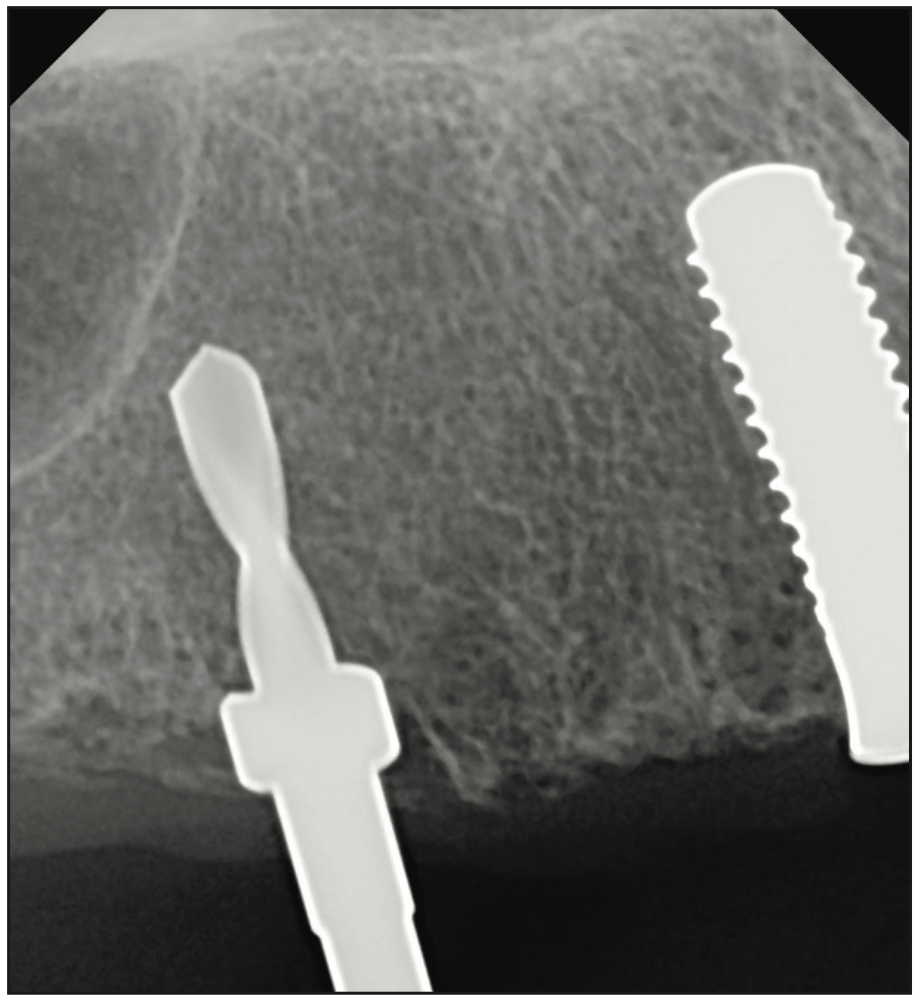
Figure 6. Intraoperative radiograph to confirm implant parallelism and also showing the use of drill stoppers for depth control.
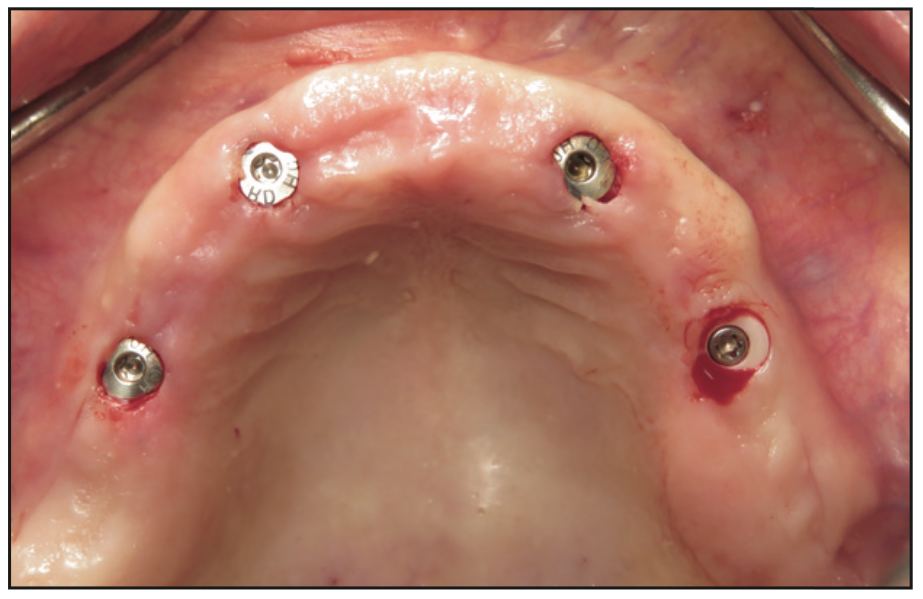
Figure 7. Clinical photo immediately after implant placement showing the minimally invasive approach.
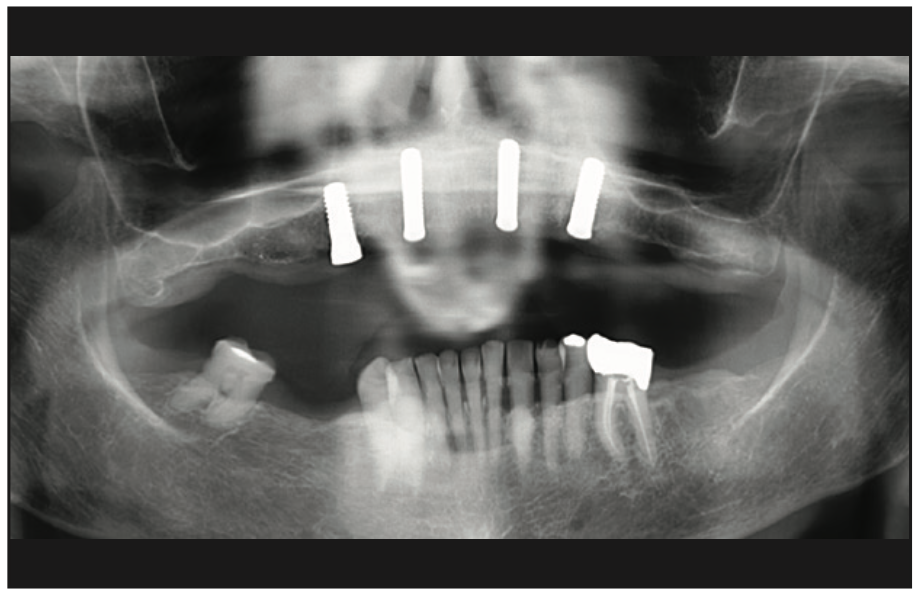
The clinical examination revealed a fully edentulous maxillary ridge with adequate keratinized tissue (Figure 3). A panoramic radiograph was taken to evaluate the location of maxillary sinuses and the available bone for adequate anteroposterior positioning of the implants (Figure 4). Available treatment options were discussed, and the patient chose the removable overdenture due to finances and less stringent hygiene requirements in comparison to a fixed implant bridge. The surgery was carried out freehand with local anesthesia. Since the patient had adequate keratinized tissue, a mucotome was used to access the bone instead of laying an extended cross-arch flap. Implant osteotomy was done using copious irrigation and manufacturer protocols, and 4 Straumann Pure 2-piece ceramic implants (Straumann) (Figure 5) were placed in the maxillary arch. Intraoperative radiographs were taken to confirm the positioning and angulation of the implants (Figure 6). Final insertion torque of the implants was 35 to 40 N/cm2, and implant stability quotient (ISQ) values of 60 to 78 were measured using the Osstell Beacon device (W&H Dentalwerk). Cover screws were placed over 3 of the 4 implants, and a short healing abutment was placed on the right posterior implant (Figure 7). The implants were left to heal undisturbed. The postoperative panoramic radiograph shows adequate spacing and parallelism of the implants (Figure 8).

Figure 9. The PUREloc plan abutment was used to determine the correct height of the final locator-type abutments.
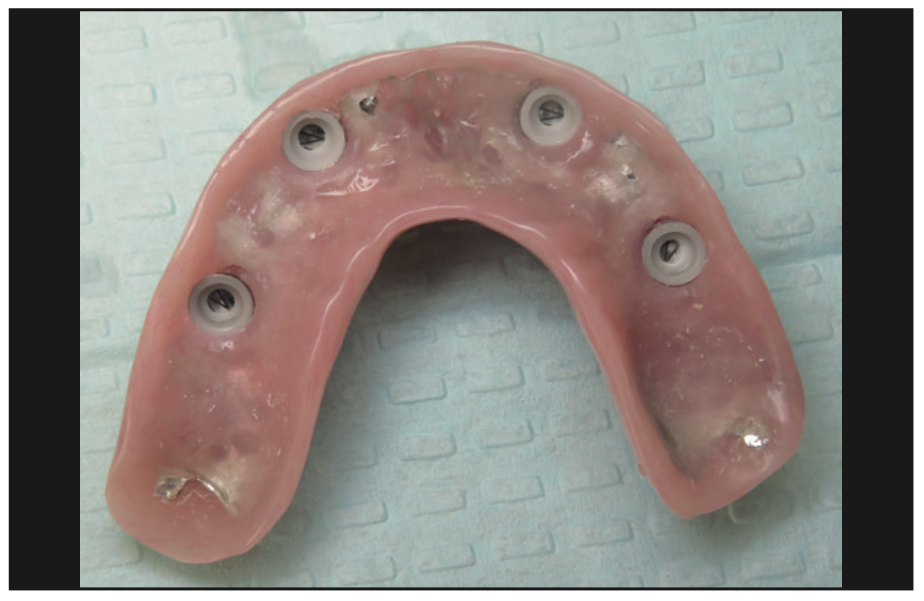
Figure 12. Intaglio of the overdenture showing the PUREloc housings with white (light, 750-g retention) inserts.
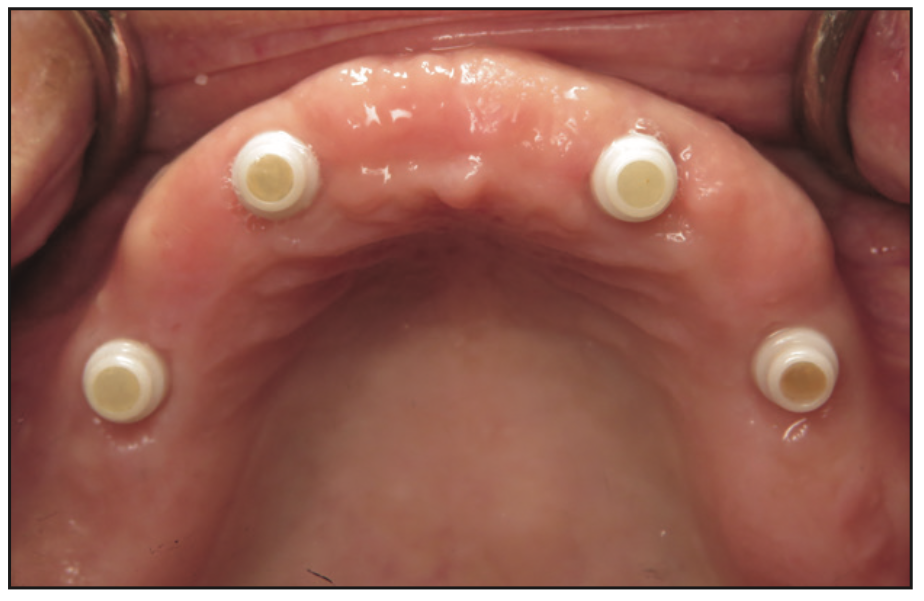
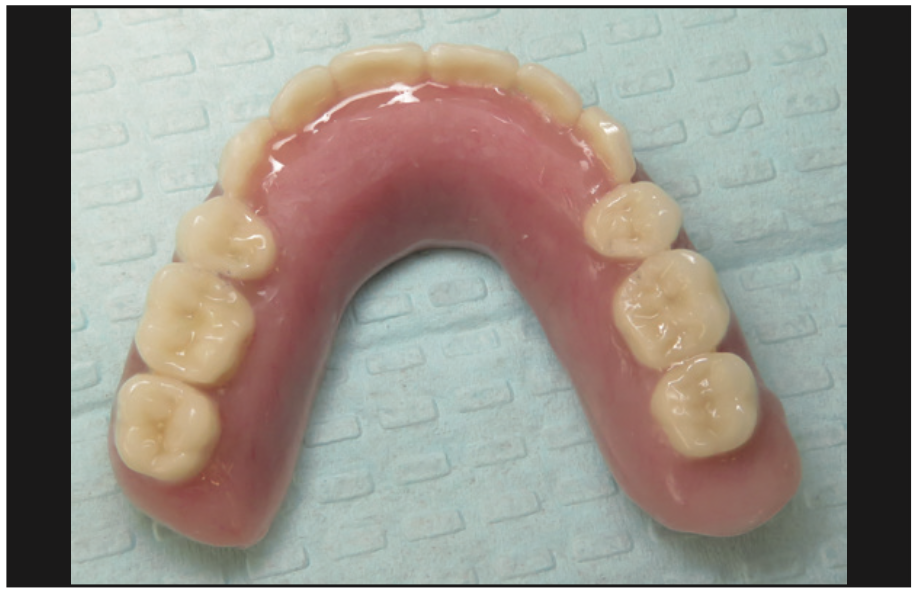
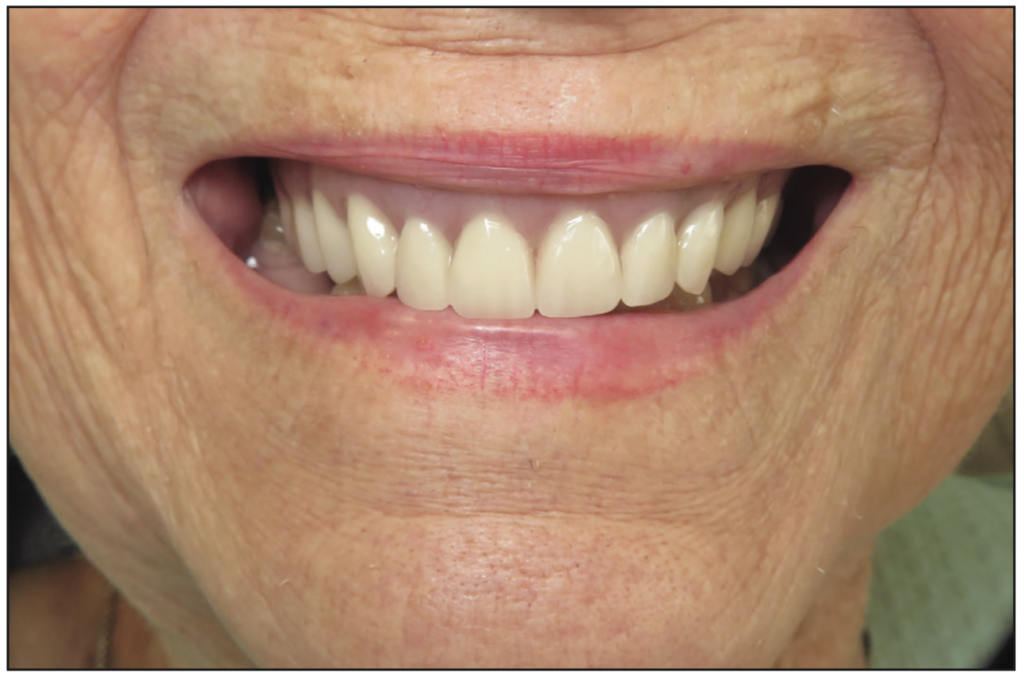
The implants were uncovered 4 months after placement, and healing abutments were placed. The ISQ values showed an increase from 71 to 78 on all implants. The gingival tissue height was measured using the PUREloc plan abutment (Figure 9), which allows for the determination of the exact height of the locator abutment to be used. Two weeks after uncovering, the PUREloc abutments were positioned (Figure 10), and impressions were taken to initiate the prosthetic phase. A palateless maxillary implant-supported overdenture (Figures 11 and 12) was fabricated and delivered. The patient was happy with the newfound retention, stability, and aesthetics of her overdenture (Figure 13), as well as the ability to have an open palate and better taste food.
DISCUSSION
Ceramic implants are being requested at an exponential pace by patients. The demand for ceramic implants is primarily driven by patients. After a decade and a half of presence in the United States and more than a dozen FDA-approved and commercially available systems, we are just now seeing a rise in the demand for ceramic implants from dentists themselves. The largest players in the dental implants industry, namely Straumann, Nobel Biocare, Neodent, and Keystone, have either added ceramic implants to their portfolios by means of licensing and distribution or, in the case of Straumann and Neodent, are manufacturing their own ceramic implants. The Straumann Pure portfolio of 2-piece ceramic implants is currently limited to implants 4.1 mm in diameter. The authors believe that having wider implants of 5 mm and greater diameters will allow for better strength, support, and favorable load distribution for edentulous solutions. That said, there are other commercially available 2-piece ceramic implants that are 5-plus millimeters in diameter and have locator-type solutions.
CONCLUSION
The evolution of zirconia materials has allowed for a better prosthetic variety, including the fabrication of LOCATOR-type abutments that allow for full-arch solutions. This case demonstrates the successful use of ceramic implants in order to rehabilitate a fully edentulous maxillary arch. As material science advances, we also expect the production of multi-unit-type abutments that will allow for an abutment-level, fixed, full-arch solution (All-on-X) with ceramic implants. That said, since the majority of 2-piece ceramic implants currently available are tissue-level, a fixture-level All-on-X solution is also currently possible on ceramic implants.
REFERENCES
1. Fleming E, Afful J, Griffin SO. Prevalence of tooth loss among older adults: United States, 2015–2018. NCHS Data Brief. 2020;(368):1-8. https://www.cdc.gov/nchs/data/databriefs/db368-h.pdf
2. Yao CJ, Cao C, Bornstein MM, et al. Patient-reported outcome measures of edentulous patients restored with implant-supported removable and fixed prostheses: A systematic review. Clin Oral Implants Res. 2018;29(Suppl 16):241–54. doi:10.1111/clr.13286
3. Neves FD, Mendonça G, Fernandes Neto AJ. Analysis of influence of lip line and lip support in esthetics and selection of maxillary implant-supported prosthesis design. J Prosthet Dent. 2004;91(3):286-8. doi:10.1016/j.prosdent.2003.12.006
4. Balaguer J, Ata-Ali J, Peñarrocha-Oltra D, et al. Long-term survival rates of implants supporting overdentures. J Oral Implantol. 2015;41(2):173–7. doi:10.1563/AAID-JOI-D-12-00178
5. Di Francesco F, De Marco G, Sommella A, et al. Splinting vs not splinting four implants supporting a maxillary overdenture: A systematic review. Int J Prosthodont. 2019;32(6):509–18. doi:10.11607/ijp.6333
6. Slot W, Raghoebar GM, Vissink A, et al. A systematic review of implant-supported maxillary overdentures after a mean observation period of at least 1 year. J Clin Periodontol. 2010;37(1):98-110. doi:10.1111/j.1600-051X.2009.01493.x
7. Sadowsky SJ. Treatment considerations for maxillary implant overdentures: a systematic review. J Prosthet Dent. 2007;97(6):340–8. doi:10.1016/S0022-3913(07)60022-5
8. Schierano G, Mussano F, Faga MG, et al. An alumina toughened zirconia composite for dental implant application: in vivo animal results. Biomed Res Int. 2015;2015:157360. doi:10.1155/2015/157360
9. Kelly JR, Denry I. Stabilized zirconia as a structural ceramic: an overview. Dent Mater. 2008;24(3):289–98. doi:10.1016/j.dental.2007.05.005
10. Monzavi M, Zhang F, Meille S, et al. Influence of artificial aging on mechanical properties of commercially and non-commercially available zirconia dental implants. J Mech Behav Biomed Mater. 2020;101:103423. doi:10.1016/j.jmbbm.2019.103423
11. Osman RB, Payne AG, Duncan W, et al. Zirconia implants supporting overdentures: a pilot study with novel prosthodontic designs. Int J Prosthodont. 2013;26(3):277–81. doi:10.11607/ijp.2903
12. Osman RB, Swain MV, Atieh M, et al. Ceramic implants (Y-TZP): are they a viable alternative to titanium implants for the support of overdentures? A randomized clinical trial. Clin Oral Implants Res. 2014;25(12):1366–77. doi:10.1111/clr.12272
ABOUT THE AUTHORS
Dr. Boyer graduated from the University of California, Los Angeles (UCLA) School of Dentistry in 2008. He completed 2 hospital-based GPR training programs at Cedars-Sinai Medical Center in Los Angeles and in the VA Healthcare system, where he received advanced training in oral and maxillofacial surgery, complex and comprehensive treatment planning, and the placement and restoration of dental implants. He has been in private practice in Los Angeles since 2010. Dr. Boyer is a faculty member at the UCLA School of Dentistry, where he teaches dental students as well as the residents in the Advanced Education in General Dentistry program. In 2020, he was invited to be one of 4 investigators in a 5-year, worldwide multicenter ceramic implant osseointegration and stability study led by the International Academy of Ceramic Implantology (IAOCI) and the Zirconia Implant Research Group. Dr. Boyer is active in multiple dental implant organizations and was recently appointed as a board member of the IAOCI. He can be reached at dentist@ucla.edu.
Dr. Noumbissi obtained his DDS degree from Howard University in Washington, DC. He attended the prestigious Loma Linda University full-time graduate program in implant dentistry. There, he received 3 years of formal training in dental implantology, which culminated with a certificate in implant dentistry and an MS degree in implant surgery. He is a clinical and experimental researcher and author and has published book chapters and articles in implant dentistry with a focus on ceramic implants in peer-reviewed dental journals. He is a visiting professor at the University of Milan, an adjunct professor the University of Chiety-Pescara in Italy, and a visiting researcher in the ceramic materials department at INSA Lyon in France. Dr. Noumbissi is the founder and current president of the International Academy of Ceramic Implantology and a Fellow and an ambassador of the Clean Implant Foundation. Dr. Noumbissi has extensive experience with ceramic implants, and his opinions and expertise are often sought both by clinicians and manufacturers. His practice is located in Silver Spring, Md. He can be reached at sammy@iaoci.com.
Disclosure: Dr. Boyer has lectured for Osstell. He did not receive any financial compensation for this article. Dr. Noumbissi reports no disclosures.