Columbia University’s Salivary Gland Center (SGC) serves as a referral base for metropolitan New York City patients with salivary gland disease and/or salivary secretory dysfunction. Since its establishment in 1988, the SGC has seen approximately 6,000 patients with varied objective and/or subjective signs of salivary gland disease. An accurate diagnosis for these patients requires a range of techniques, with the individual diagnostic approach dictated by the clinical picture. Besides a thorough history and physical examination, differential diagnosis incorporates (when indicated) an evaluation of salivary volume, serology, imaging, and biopsy. Imaging procedures used by the SGC may include standard radiography, sialography, ultrasound, computerized tomography (CT) scanning, magnetic resonance imaging (MRI), and radioisotope studies. It is only after the work-up is completed that a confident diagnosis can be made.
The SGC has observed that many of its patients do not have problems associated with their salivary glands and/or saliva. These cases are false positives, and they represent a variety of subjective complaints and/or objective conditions (Table). With the goal of alerting the profession to the existence of these pitfalls, a review of these false-positives follows, with the understanding that it is strictly limited only to those patients examined in the SGC. Because of the numerous and varied conditions seen in the SGC that mimic salivary gland disease, this review will only highlight their significant symptomatology and major means of differentially diagnosing these conditions. Improvement in diagnostic skills will evolve and will act to avoid needless referrals and therapy. Part 1 of this review will examine somatoform disease, neuromuscular dysfunction, masseteric hypertrophy, lymphadenopathy, and dental etiologies. Part 2 will examine paraglandular opacities, fascial space infections, proliferating masses of the ramus, and paraglandular soft-tissue pathology.
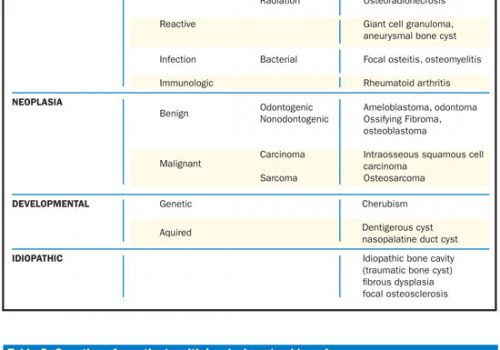
| Table. False Positives in Salivary Gland Disease. |
|
I. Somatoform disease |
SOMATOFORM DISEASE
Unexpectedly, a high percentage of patients (approximately 50% of patients seen in the SGC) have no discernible organic salivary gland disease and/or secretory disturbance. Their complaint appears to be somatoform in origin, wherein a patient’s psychologic problems, usually depression, are manifested by a physical complaint with no organic basis.1,2 Many of these patients are caught in a self-perpetuating cycle in which symptoms of common minor problems are magnified to such an extent that they are interpreted as being serious disorders.3
Most complaints are associated with xerostomia, excessive salivation, or sialorrhea. Others are odd and somewhat bizarre. They include dryness or excessive salivation limited only to one area, a need to constantly expectorate or swallow due to an imagined salivary excess, salivary spraying during speech, and continuous drooling when in fact no such events are observed during the prolonged examination process. Patients have been seen because they believe that their saliva is too thick, gritty, or slimy, and that it sticks to specific oral areas. Visually, it is apparent that their saliva is aqueous and clear. Frothy saliva is also a frequent patient concern. The explanation for frothiness rests in the fact that when saliva lies in the mouth floor, speech and its related oral muscular activity cause salivary aeration, giving saliva a foamy appearance (Figure 1). In somatoform patients, bitter-tasting saliva,4 often limited to a defined oral area, also serves as an impetus for a visit. Nevertheless, the clinician should be aware that some medications and systemic diseases can alter taste, and their role must be determined in each patient.5,6
 |
| Figure 1. Normal saliva that has been aerated by oral muscular activity. |
Common denominators in the somatoform patients include histories of emotional disturbance (depression) and past or present use of psychotherapeutic medications. The patients may blame xerostomia for frequent night awakenings when in reality the mental state is the cause. Many times patients bring very detailed written chronologic histories of their symptoms, numerous medical visits, and medications. Their multiple medical consultations have not solved their problem and have prompted the visit to the SGC. Questioning often reveals that some oral event, or dental care of some kind, was the precipitating factor for the problem. Such patients can be hostile and litigious, placing blame on the dentist. Medication-related xerostomia can also serve as an instigator for and accentuator of their complaint. In reality, such situations only serve to focus the patients’ attention on their mouth. Contributory causes include stressful work and social or family situations whose details can be elicited during a thorough interviewing process.
Concurrently, many patients will demonstrate other oral conditions that may have psychogenic aspects. A complete history and examination can uncover the presence of idiopathic burning mouth,7,8 bruxing and clenching, and unusual patterns of pain for which no organic reason can be discerned.9
In determining salivary volume, the SGC uses the previously established criteria for measuring stimulated flow rates with a Carlsen-Crittenden collector (normally 0.4 to 1 mL/minute per parotid gland).10 When saliva is stimulated and volume measured in patients with disturbed perceptions of xerostomia or sialorrhea, a normal amount is obtained. In medication-related xerostomia, stimulation will also result in a normal return because it overrides the drug’s anticholinergic effect on the resting gland. Conversely, the true pathology-related xerostomic patient will produce reduced saliva both when the salivary gland is at rest and when activated.
The frequently observed triad of neurosis, a precipitating oral event, and the presence of bizarre and unsubstantiated salivary complaints in the absence of any salivary gland pathology point to a diagnosis of somatoform disorder.3 In these patients, an understanding of the origin of their symptoms can lead to its reduction or disappearance. Often patients require psychiatric consultation, but frequently resist the suggestion for such help.
NEUROMUSCULAR DYSFUNCTION
Drooling patients have an inability to manage normal salivary volume, while sialorrhea patients produce a true excessive salivary volume. The drooling problem originates from a neuromuscular dysfunction that impedes normal swallowing and prevents management of saliva whose volume proves to be normal when calibrated.
Swallowing is a complex process. Once masticated, food reaches the posterior oral cavity, and an involuntary swallow reflex is initiated involving both the pharynx and larynx.11,12 A wide variety of disorders can cause swallowing dysfunction. Patients may have amyotrophic lateral sclerosis (ALS), Parkinson’s disease (PD), stroke, demyelinating diseases, poliomyelitis, or a muscular dystrophy.13,14 Problems have also been noted in patients who have received radiation therapy for oral/neck cancer, had radical oral/neck surgery, or are severely mentally retarded.15 In such patients, saliva will accumulate in the anterior mouth floor because it cannot be transported backward in the oral cavity due to altered neuromuscular function. The salivary accumulation is further amplified by dysphagia. Saliva collecting in the anterior mouth floor, aided by a failure to make a functional lip seal and a tendency of the head to lean forward, results in the patient’s propensity to drool.16
Therapeutic management of the drooling includes the surgical repositioning of salivary ducts into the posterior oral cavity, the use of antisialogogic medications, low-dosage irradiation of the salivary glands, surgical interruption of the parasympathetic nerve supply to the salivary glands, and cricopharyngeal myotomy to relieve any muscle spasm that prevents free movement of the food bolus into the esophagus.17
MASSETERIC HYPERTROPHY
Many patients referred to the SGC are found to have masseteric hypertrophy (MH) rather than a true parotid enlargement. The masseter is a thick quadrate masticatory muscle arising from the zygomatic arch and inserting into the lateral aspect and angle area of the mandibular ramus where the facial contour normally tapers.
MH can be caused by constant bruxing, clenching, or heavy gum chewing. Emotional stress seems to be an underlying factor in many cases. With masseteric enlargement, the patient’s face takes on a characteristic rectangular configuration (Figure 2a). Conversely, an enlarged parotid, because of its higher anatomic location adjacent to the ear, tends to accentuate the ovality of the face. When the palm of the hand is placed on the facial swelling and the patient clenches, the previously enlarged muscle becomes even larger and markedly displaces the hand laterally. Because the enlargement is asymptomatic and slow in development, the patient often is unaware of its presence. The condition is primarily seen in young adults with good dentitions. It is rarely seen in older patients because dental deterioration leads to pain during heavy occlusion, and full activation of the masseters is discouraged. The hypertrophy is usually bilateral but not necessarily symmetrical. Unilateral muscle enlargement can also develop18 (Figure 2b). Orally, tooth attrition from bruxing or clenching may be present.
 |
| Figure 2a. Bilateral masseteric hypertrophy (arrows). |
Radiographically, in the angle of the mandible, which is the region of the muscle’s insertion, bony hyperplasia develops as a result of the stimulatory effect on bone from increased muscle tension.19 A change in the gonial angle also can be anticipated. Cephalometric studies have demonstrated gonial angles averaging 106º (normal 124º to 126º) in patients with MH.20 If needed, the CT scan and MRI can be helpful in diagnosing MH.21
 |
| Figure 2b. CT scan. Unilateral right (R), masseter muscle (M) hypertrophy. Compare right side to left side. |
Other than increased muscle fiber length and diameter, histologic findings reveal no abnormalities.20 Therefore, therapy is conservative and primarily revolves around informing and reassuring the patient. Muscle relaxants, psychiatric care, behavioral modification, construction of an occlusal guard, and surgical muscle reduction are therapeutic options. Botulinum toxin injections have been used to prevent neurologic stimulation of the muscle and lead to muscle atrophy.18
LYMPHADENOPATHY
An understanding of anatomy, plus a knowledge of the many pathologic entities that can lead to lymphadenopathy, serve to differentiate accurately lymphadenopathy from sialadenopathy.
The etiology of lymphadenopathy has proven to be extremely varied. The most common cause for the enlarged lymph node seen by the SGC is bacterial lymphadenitis. Most infected areas in the oral cavity and on the side of the face are drained by afferent lymphatic channels leading to the submandibular nodes. Pain and swelling of these nodes are the end result.22 An infected tooth (Figures 3a and 3b) or mucosal condition are the frequent causes. Besides bacteria, viral, mycotic, and protozoan organisms also can cause lymphadenopathy.22,23
 |
 |
| Figure 3a. Infected molars with fistulous tract (parulis) present (arrow). | Figure 3b. Submandibular lymphadenopathy secondary to infected molars seen in Figure 3a. |
Many other causes for lymphadenopathy exist. Granulomatous diseases can involve lymph nodes in the region of the salivary gland (Figures 3c and 3d). Autoimmune diseases often involve the salivary glands, but lymphadenopathy due to that cause can stand alone without a salivary gland linkage.22
 |
 |
| Figure 3c. Lymphadenopathy (arrow) from sarcoidosis. | Figure 3d. Microscopic view of sarcoidal granulomas (G) in lymph node of patient seen in Figure 3c (hematoxylin-eosin stain; original magnification 120x). |
Lymph nodes are frequently a foci for metastatic malignancies, with the metastases that involve the submandibular or submental nodes often originating from the oral cavity. Because of the nodes’ anatomic proximity to the salivary glands, patients with these lymphadenopathies have occasionally been referred with a presumed salivary gland problem (Figures 3e and 3f).
 |
 |
| Figure 3e. Invasive squamous cell carcinoma of the mouth floor (arrow). | Figure 3f. Submandibular lymphadenopathy from the metastatic oral carcinoma seen in Figure 3e. |
Although metastases from the oral cavity are the most common cause for malignant lymphadenopathy, primary lymphomas also can occur in lymph nodes in the submandibular/cervical area and usually represent Hodgkin’s disease. Non-Hodgkin’s lymphomas may also develop.24 A patient with a T-cell lymphoma in the parotid area has been reported.25
Clinical criteria, involving a thorough history and physical examination of the patient, must be utilized in diagnosis in conjunction with knowledge of lymphatic drainage patterns. The sudden onset of swelling and tenderness in a node that is movable and circumscribed clearly suggests the presence of a lymphadenitis. Neoplastic nodes tend to be persistent, painless, fixed, grow, and require a systemic investigation. A simple method to differentiate a lymphadenopathy from a sialadenitis is to observe whether expressed saliva exiting from the suspected gland’s duct is cloudy or clear. Cloudiness suggests the presence of pus associated with a sialadenitis, while clear saliva implies an extraglandular process.
A CT scan or MRI is most helpful in indicating the presence of a lymphadenopathy. Biopsy, whether it be fine needle aspiration biopsy or tissue biopsy, usually proves to be a most significant aspect of the diagnostic work-up.
DENTAL ETIOLOGIES
Saliva is recognized to have an important protective effect in preventing caries.10,26-29 Because this beneficial effect of saliva is common knowledge, many patients with extensive dental damage caused by diverse conditions are referred to the SGC. The tentative assumption that serves as the impetus for the referral is that some deficiency in salivary volume or quality is the causative factor for the dental caries. Patients with caries related to a reduction in saliva associated with Sjogren’s syndrome or radiation are readily recognized and treated appropriately. Other patients seen do not seem to have a xerostomia sufficient enough to cause increased caries rates. Intraorally, normal mucosal moistening is evident in the false-positive group with dental caries. Milking of each salivary gland will produce a normal volume of aqueous clear saliva exiting from the duct orifice. Of interest, patients with gastroesophageal reflux disease regurgitate gastric acids and will develop dental erosions.30,31
Diet
Salivary gland disease with secretory dysfunction in patients with extensive caries can be ruled out simply by observing salivary flow from the duct orifice, the observation of a normally moist mucosa, and the help of a xerostomia questionnaire.32-34 Usually, questioning will elicit information pointing to diet as the culpable factor.35,36 Patients with an extremely high sucrose intake originating from large quantities of soft drinks and fruit juices, constant sucking on mints, and continuous use of bubblegum have all been seen (Figure 4a).
 |
| Figure 4a. Rampant cervical caries secondary to habitual sucking on mints. |
Treatment requires patient reassurance, diet modification, good oral hygiene, and dental rehabilitation.
Abrasion
Abrasion is the wearing away of tooth substances by incorrect brushing techniques, bruxism, or foreign objects. Excessive toothbrushing, particularly in an inappropriate manner and with a hard-bristled brush, can result in a loss of dental enamel.37 The enamel loss is most marked buccally in the area of the dental arch’s curvature (the cuspid and bicuspid regions), because it receives the focus of the brush’s force during toothbrushing (Figure 4b).
A prolonged history of bruxism can cause pronounced dental damage to the occlusal surfaces of teeth38 (Figure 4c). Saliva does not play a role in such dental wear, but patients have been referred to the SGC with the false supposition that salivary dysfunction was the cause.
 |
 |
| Figure 4b. Toothbrush trauma with abfraction. | Figure 4c. Bruxism causing marked abrasion of bicuspid and molar teeth |
Regardless of the cause, treatment of abrasion includes patient understanding, modification of the causative activity, and restorative dentistry.
CONCLUSION
Numerous and varied conditions have been seen in the SGC that mimic salivary gland disease. Appropriate diagnostic skills will act to avoid needless referrals and therapy. Part 1 of this review has examined somatoform disease, neuromuscular dysfunction, masseteric hypertrophy, lymphadenopathy, and dental etiologies. Part 2 will examine paraglandular opacities, fascial space infections, proliferating masses of the ramus, and paraglandular soft-tissue pathology.
References
1. Barsky AJ, Borus JF. Functional somatic syndromes. Ann Intern Med. 1999;130:910-921.
2. Simon GE, Van Korff M, Piccinelli M, et al. An international study of the relation between somatic symptoms and depression. New Engl J Med. 1999;341:1329-1335.
3. Votta TJ, Mandel L. Somatoform salivary complaints. Case reports. N Y State Dent J. 2002;68:22-26.
4. Brown C. The parotid puzzle: a review of the literature on human salivation and its applications to psychophysiology. Psychophysiology. 1970;7:65-85.
5. Deems DA, Doty RL, Settle RG, et al. Smell and taste disorders, a study of 750 patients from the University of Pennsylvania Smell and Taste Center. Arch Otolaryngol Head Neck Surg. 1991;117:519-528.
6. Osaki T, Ohshima M, Tomita Y, et al. Clinical and physiological investigations in patients with taste abnormality. J Oral Pathol Med. 1996;25:38-43.
7. Grushka M, Sessle BJ. Burning mouth syndrome. Dent Clin North Am. 1991;35:171-184.
8. Rojo L, Silvestre FJ, Bagan JV, et al. Psychiatric morbidity in burning mouth syndrome. Psychiatric interview versus depression and anxiety scales. Oral Surg Oral Med Oral Pathol. 1993;75:308-311.
9. Aghabeigi B, Feinmann C, Harris M. Prevalence of post-traumatic stress disorder in patients with chronic idiopathic facial pain. Br J Oral Maxillofac Surg. 1992;30:360-364.
10. Mandel ID. Sialochemistry in diseases and clinical situations affecting salivary glands. Crit Rev Clin Lab Sci. 1980;12:321-366.
11. Sochaniwskyj AE, Milner M, Kenny DJ. Oral motor functioning, frequency of swallowing and drooling in normal children and in children with cerebral palsy. Arch Phys Med Rehabil. 1986;67:866-874.
12. Dua KS, Ren J, Bardan E, et al. Coordination of deglutitive glottal function and pharyngeal bolus transit during normal eating. Gastroenterology. 1997;112:73-83.
13. Ott DJ, Pikna LA. Clinical and videofluoroscopic evaluation of swallowing disorders. AJR Am J Roentgenol. 1993;161:507-513.
14. Dray TG, Hillel AD, Miller RM. Dysphagia caused by neurologic deficits. Otolaryngol Clin North Am. 1998;31:507-524.
15. Logemann JA, Bytell DE. Swallowing disorders in three types of head and neck surgical patients. Cancer. 1979;44:1095-1105.
16. Coates C, Bakheit AM. Dysphagia in Parkinson’s disease. Eur Neurol. 1997;38:49-52.
17. McKenna JA, Dedo HH. Cricopharyngeal myotomy: indications and technique. Ann Otol Rhinol Laryngol. 1992;101:216-221.
18. Mandel L, Tharakan M. Treatment of unilateral masseteric hypertrophy with botulinum toxin: case report. J Oral Maxillofac Surg. 1999;57:1017-1019.
19. Guggenheim P, Cohen L. The nature of masseteric hypertrophy. Arch Otolaryngol. 1961;73:15-28.
20. Ahlgren J, Omnell KA, Sonesson B, et al. Bruxism and hypertrophy of the masseter muscle. A clinical, morphological and functional investigation. Pract Otorhinolaryngol. 1969;31:22-29.
21. Seltzer SE, Wang AM. Modern imaging of the masseter muscle: normal anatomy and pathosis on CT and MRI. Oral Surg Oral Med Oral Pathol. 1987;63:622-629.
22. Fletcher RH, Malamud SC, Slap G, et al. When you find lymphadenopathy. Patient Care. 1992;26:83-103.
23. Mandel L, Surattanont F, Miremadi R. Cat-scratch disease: considerations for dentistry. J Am Dent Assoc. 2001;132:911-914.
24. Mehle ME, Kraus DH, Wood BG, et al. Lymphoma of the parotid gland. Laryngoscope. 1993;103:17-21.
25. Mandel L, Surattanont F, Dourmas M. T-cell lymphoma in the parotid region after cardiac transplant: case report. J Oral Maxillofac Surg. 2001;59:673-677.
26. Woltgens JH, Bervoets TJ, Witjes F, et al. Effects of post-eruptive age on Ca and P loss from human enamel during demineralization in vitro. Arch Oral Biol. 1981;26:721-725.
27. Slomiany BL, Murty VL, Zdebska E, et al. Tooth surface-pellicle lipids and their role in the protection of dental enamel against lactic-acid diffusion in man. Arch Oral Biol. 1986;31:187-191.
28. Mandel ID. The role of saliva in maintaining oral homeostasis. J Am Dent Assoc. 1989;119:298-304.
29. Jensen ME. Diet and dental caries. Dent Clin North Am. 1999;43:615-633.
30. Dashan A, Patel H, Delaney J, et al. Gastroesophageal reflux disease and dental erosion in children. J Pediatr. 2002;140:474-478.
31. Munoz JV, Herreros B, Sanchiz V, et al. Dental and periodontal lesions in patients with gastro-oesophageal reflux disease. Dig Liver Dis. 2003;35:461-467.
32. Fox PC, Busch KA, Baum BJ. Subjective reports of xerostomia and objective measures of salivary gland performance. J Am Dent Assoc. 1987;115:581-584.
33. Nederfors T, Isaksson R, Mornstad H, et al. Prevalence of perceived symptoms of dry mouth in an adult Swedish population—relation to age, sex and pharmacotherapy. Community Dent Oral Epidemiol. 1997;25:211-216.
34. Locker D. Dental status, xerostomia and the oral health-related quality of life of an elderly institutionalized population. Spec Care Dentist. 2003;23:86-93.
35. Burt BA, Pai S. Sugar consumption and caries risk: a systematic review. J Dent Educ. 2001;65:1017-1023.
36. Moynihan PJ. Dietary advice in dental practice. Br Dent J. 2002;193:563-568.
37. Addy M, Hunter ML. Can tooth brushing damage your health? Effects on oral and dental tissues. Int Dent J. 2003;53(suppl 3):177-186.
38. Khan F, Young WG, Daley TJ. Dental erosion and bruxism. A tooth wear analysis from south east Queensland. Aust Dent J. 1998;43:117-127.
Dr. Mandel is the director of the Salivary Gland Center and assistant dean and clinical professor in the Section of Hospital Dentistry, Division of Oral and Maxillofacial Surgery, Columbia University School of Dental and Oral Surgery, New York – Presbyterian Hospital (Columbia campus) in New York City. He can be reached at (212) 305-9982.