During the 20th century, significant advances were made worldwide in health care and the biological sciences. Key milestones included the discovery of penicillin by Alexander Fleming in 1928, heralding in the antibiotic era where it became possible to treat debilitating and potentially fatal infections with an increasing array of antibiotics.1 Vaccine developments and their use globally in immunization programs have also resulted in the prevention of many diseases by micro-organisms transmitted by direct contact, indirect contact, bloodborne, airborne, waterborne, droplet, and vector routes.2,3 However, existing diseases have also re-emerged as threats, such as tuberculosis due to multi-drug resistance and measles due to vaccine hesitancy.4,5 The emergence of new diseases, including HIV/AIDS; H1N1; SARS; MERS; and, most recently, COVID-19, have also highlighted vulnerabilities. Further, the risk of zoonotic diseases, epidemics, and pandemics is increasing due to burdensome population growth forcing people to live in closer proximity with animals and due to inappropriate environments.6 It is also abundantly clear that transmission is advanced by an interconnected world.
DISEASE TRANSMISSION IN HEALTHCARE SETTINGS
Modes of transmission for healthcare workers and patients in healthcare settings include direct contact, indirect contact with contaminated surfaces or objects (fomites), droplets, and bloodborne and airborne routes.7,8 Disease may occur when a sufficient level of a given pathogen is present to result in an infectious dose.7 In hospitals, 1 in 25 patients are impacted by at least one hospital-acquired infection (HAI).9 Many HAIs are caused by antibiotic-resistant micro-organisms, often referred to as “superbugs,” and may lead to sepsis or death.10 In the dental setting, confirmed diagnoses of acquired infections among patients and/or dental healthcare personnel have resulted from water in contaminated dental unit waterlines, failure to adhere to the requirements of the Bloodborne Pathogens Standard, and incorrect/inadequate reprocessing of contaminated instruments.7,8,11 Other risks include contaminated clinical contact surfaces, direct contact, and airborne transmission.
AIRBORNE TRANSMISSION
Examples of micro-organisms with airborne transmission include the measles virus and Mycobacterium tuberculosis, which are both highly infectious, and Candida auris.12-15 Airborne spread of SARS was confirmed more than a decade ago.16 In air sampling studies of hospital air where infected patients were staying, PCR was used to detect micro-organisms.17 Collectively in these studies, sampled air had contained the varicella-zoster virus, measles virus, M tuberculosis, influenza viruses, respiratory synctial virus, rhinovirus, adenovirus, Mycoplasma pneumoniae, and other micro-organisms. For many, the particles were <5 μm in size. There was also evidence of airborne transmission during the SARS-CoV outbreak.18 For some micro-organisms, while one mode of transmission may dominate, other modes of transmission also occur: for example, MRSA (which can be transmitted by direct contact and indirect contact with fomites), varicella-zoster, and Pseudomonas aeruginosa.19-21 The latest was SARS-CoV-2, which is now generally accepted to mainly involve airborne transmission, with far fewer cases associated with close-contact droplet transmission or contaminated surfaces.
Particle Size and Behavior
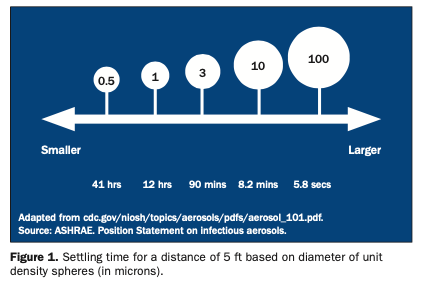
Different understandings exist with respect to airborne transmission. Aerosols contain particles ≤50 μm in a gas—in the current context, in air. Infectious aerosols contain pathogens within particles in the air. Larger particles are generally believed to behave ballistically and to settle out rapidly in close proximity. This includes spatter, which contains particles >50 μm and droplets >20 μm in aerosols.16,22 However, in a study on respiratory exhalation, it was found that droplets between 60 and 100 μm were carried farther than 6 m away.23 In addition, when droplets rapidly dehydrate in the air, this creates airborne droplet nuclei, which are largely ≤5 μm but may be as large as 10 μm.16 Small particles can move with airflow, remain airborne longer, and travel farther than larger particles.24 The time it takes for particles to settle varies based on particle size, airflow, and the height of the area in which settling occurs. Studies use the diameter of unit density spheres to assess settling time that involves the same velocity as for the actual particle being investigated (Figure 1).
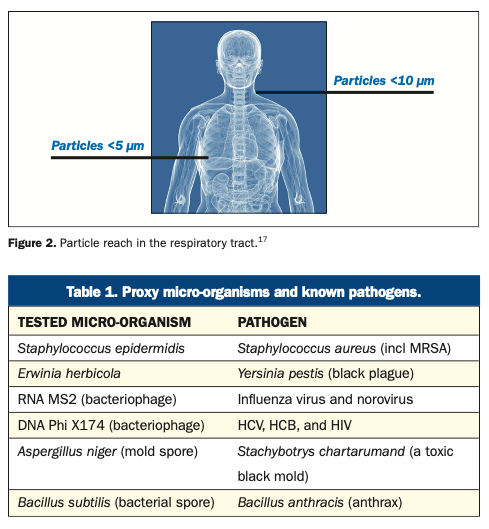
Furthermore, while particles passing in turbulent air close to a horizontal surface can settle, other particles will continue to be disturbed and remain airborne for long periods of time.24 In addition, it is possible for particles settled on surfaces to become airborne. Whether or not this occurs depends on particle size, adhesion to a surface, and the energy and airflow reaching the space.24 A recent report noted that micro-organisms on floors in hospital settings could become resuspended into the air, such as when walked on, with a potential for transmission and contamination of surfaces.25 The report also cited earlier studies showing the contamination of floors with Clostridioides difficile, vancomycin-resistant enterococci, and MRSA. Particle size also determines how deep a pathogen can reach in the respiratory tract, with smaller aerosol particles reaching deeper into the lungs. In studies analyzing cough and breath aerosols, M tuberculosis, P aeruginosa, and numerous viruses were found in particles <5 μm in size.17 Particles <10 μm readily reach below the glottis in the larynx; those <5 μm are readily inhaled into the lower respiratory tract16,17,26 (Figure 2).
INFECTION CONTROL PROTOCOLS AND GUIDANCE IN THE DENTAL SETTING
The CDC provides recommendations with standard precautions and additional protocols for infection control. CDC guidance for healthcare settings during COVID-19 includes (but is not limited to) triaging patients, social distancing, source control, changes to PPE for protection, and the recommendation to select an EPA-registered hospital-level disinfectant on EPA List N.27 In the dental setting, key guidance includes the use of high-volume evacuation, rubber dams (when possible), and specific recommendations for PPE during aerosol-generating procedures. Other devices, such as extraoral suction, can also mitigate aerosolization through capture while varying in power level, dimensions, configuration, noise level, and how the captured aerosol is then handled.28 A layered approach has been recommended. This approach includes using ventilation and considering the use of adjunctive devices, specifically high-efficiency particulate air (HEPA) filtration and ultraviolet germicidal irradiation (UVGI).29 HEPA filtration and upper-room UVGI13 work continuously while rooms are occupied, providing ongoing adjunctive infection control. Newer Food and Drug Administration (FDA)-cleared medical devices are also available in the dental setting, which we will discuss later in this article.
Of note, ozone generator air purifiers are not recommended. While ozone is a germicide at high levels, it is also toxic. OSHA’s permissible level of ozone is an airborne exposure limit of 0.10 ppm (0.2 mg/m3) for an 8-hour work shift (a day of exposure). The National Institute of Occupational Safety and Health recommends an upper limit of 0.10 ppm, not to be exceeded at any time.30,31 The California Air Resource Board (CARB), which oversees air pollution control efforts in California to reach and maintain health-based air quality standards, and other governmental organizations advise against the use of such ozone generators in spaces occupied by people or animals.32,33 Based on the evidence, ozone concentrations within the permissible levels are ineffective in removing micro-organisms, as well as many odor-causing chemicals, and would need to be substantially higher to kill airborne micro-organisms.31
Air Exchanges and Ventilation
“Air changes per hour” (ACH) denotes how many times the air within a defined space is replaced, provided the air is well-mixed. The CDC defined a minimum of 6 ACH as criteria for an efficient ventilation system in 2003, while for transmission-based precautions, it states that at least 12 ACH should be ensured.34 The minimum of 6 ACH is being reviewed. As reported by the American Society of Heating, Refrigerating and Air-Conditioning Engineers (ASHRAE), the assumption in the past was that air was ideally mixed and no obstacles to airflow were present, such as a dental chair. It was also noted that it was based on an absence of “re-contamination of the room by the exhaust air.”35 The objective of increasing room ventilation is to supply more clean air, thereby reducing the concentration of contaminants with the goal of keeping this below the threshold that could cause injury/disease. It has also been shown that a relative humidity below 40% can increase the survival rate of some airborne micro-organisms and increase their transmission.35
One method to assess ventilation is through the use of carbon dioxide monitors. These determine the concentration of carbon dioxide in an area, with readings below 800 ppm recognized as a target benchmark for good ventilation. Periodic assessment of the level of carbon dioxide in a defined space provides information on the need (or not) to adjust ventilation. It can provide an early warning system when used appropriately.36
In accordance with the CDC guidance, options that include opening windows—even just slightly, when possible—aid ventilation. Fans can be used to increase the flow of outside air into the area. Another option is to consult an HVAC specialist about further opening the HVAC’s outdoor air dampers and to help ensure that the HVAC is working properly and is adjusted for optimal airflow.27 Additional methods of adjusting ventilation can be found on the CDC website.
Minimum Efficiency Reporting Value (MERV) ratings are provided for filters.37 The higher the MERV rating, the more efficient the filter. For example, the particle size efficiency of a MERV 14 filter is 76% to 84% for 0.3-μm to 1.0-μm particles and 90% or greater for 1.0-μm to 3.0-μm particles. A MERV 12 filter, on the other hand, has a particle size efficiency of 80% to 89.9% for 1-μm to 3-μm particles and 90% or greater for 3-μm to 10-μm particles. Currently, ASHRAE recommends a filter with a MERV 13 rating (at least 85% efficient at capturing particles in the 1- to 3-µm size range) for HVAC systems, while noting that a MERV 14 or better is preferred.38 It also notes that the given HVAC system must be taken into account and that it may not be possible to accommodate a MERV 13 filter. Higher efficiency typically increases the pressure drop, paradoxically, in turn, potentially reducing the airflow through the system and/or increasing energy use.37
HEPA Filtration (Adjunctive)
The CDC recommends that consideration be given to using portable HEPA filters to decontaminate air, preferably those with powered fans.27 HEPA filters can theoretically capture at least 99.97% of 0.3-μm particles, the most penetrating particle size and, therefore, the most difficult to capture compared to both larger and smaller particles.39,40 When selecting a HEPA filter, one with a high clean air delivery rate (CADR) is recommended. This defines the cubic feet of air per minute that can be handled by the filter. The device should be positioned so that air is sucked into it directionally away from patients and the clinical team, avoiding sucking contaminated air over room occupants. The room dimensions and capacity must be considered in determining the required capacity of a HEPA filter or whether more than one HEPA filter will be required.
While some devices on the market may be advertised as “HEPA-Rx,” “true HEPA” or “medical-grade HEPA” filters, these are not recognized HEPA categories.
NEWER ADJUNCTIVE TECHNOLOGIES
Many newer technologies and devices are now being marketed as adjunct devices for air decontamination. These variously offer the capture of micro-organisms and their destruction in the device and/or in the air. There are several considerations to be made before deciding whether to purchase these. First and foremost, do you need one, does it work, and is it safe? Is the device FDA-cleared or being marketed during COVID-19 under the FDA Enforcement Policy for Sterilizers, Disinfectant Devices, and Air Purifiers during the COVID-19 public health emergency or neither? Is it CARB-certified (which is needed in California)? Other factors include whether there are independent studies demonstrating efficacy against micro-organisms. Another consideration is the availability of information from studies in healthcare settings supporting efficacy. Does the device operate episodically or continuously, including safely when the room/area is occupied? Other considerations beyond the scope of this article include specifications, size, capacity, performance, ease-of-use, cost, maintenance, and ongoing support.
ActivePure Medical Guardian: The ActivePure Medical Guardian is cleared by the FDA as a Class II Medical Device and is CARB-certified. This device is portable, and the system is designed to be used in rooms of up to 3,000 ft3. The technology is an advanced form of the one developed and used in the NASA Space Program and included in the Space Foundation Technology Hall of Fame. It offers continuous air and surface decontamination and works in 2 phases using 3 technologies. The first phase utilizes a patented process that includes a UV light source in the device and titanium dioxide as a photocatalyst that is used to produce gaseous hydrogen peroxide and other oxidizers. These then exit the device and enter the air. The oxidizers interact with and disrupt bacterial and fungal cell membranes, as well as the outer shell of viruses. This leads to microbial kill. During phase 2, air is sucked into the device, along with contaminants and oxidizers present in that air. The contaminants are then ionized to become negatively charged, trapped by a positively charged filter media, and filtered by HEPA filters. In addition, the oxidizers present kill the trapped and filtered micro-organisms.
Testing Results: Studies on the technology have been conducted in laboratory and healthcare settings. Tests resulting in FDA Class II Medical Device Clearance showed a log reduction of 5 (99.999%) for airborne RNA MS2 bacteriophages in 30 minutes.
At the University of Texas Medical Branch, an independent laboratory study on the first phase of ActivePure, using only the ActivePure technology (no filters or ionizers), was conducted using SARS-CoV-2.41 The test protocol was designed to deliver 29 ft3 per minute of air movement (the lowest setting). Within 3 minutes, a ≥2.87 to ≥3.38 log reduction was found, which equates to a ≥99.87% to ≥99.96% reduction in the concentration of SARS-CoV-2. It was noted that the actual reduction may have been 99.99% or greater since the level of detection was reached.
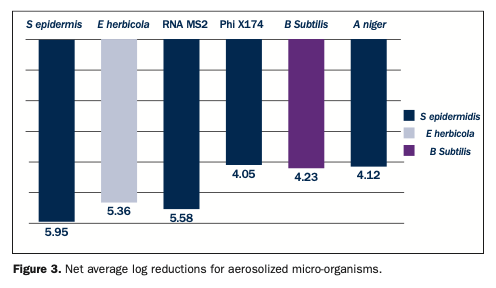
In a second independent study, the efficacy of the ActivePure Medical Guardian against aerosolized vegetative bacteria, viruses, and spores was evaluated using a sealed bioaerosol chamber in a laboratory at approximately 70.6°F and with 36% relative humidity, and inside the chamber at 75°F and 50%, respectively.41 The bioaerosol was created using a nebulizer filled with approximately 50 mL of biological stock and operated at 50 psi for 20 or 25 minutes (organism dependent). Aerosol samples were collected using 2 impingers and collected at baseline and 15-minute intervals for 90 minutes. The test micro-organisms were Staphylococcus epidermidis, Erwinia herbicola, RNA MS2 and DNA Phi X174 bacteriophages, Aspergillus niger, and Bacillus subtilis. These are proxy/surrogate micro-organisms for known pathogens, among them, respectively, Staphylococcus aureus; Yersinia pestis (black plague); influenza virus and norovirus; HCV, HCB, and HIV; Stachybotrys chartarumand (a toxic black mold); and Bacillus anthracis (anthrax) (Table 1).
Three test trials were conducted as well as a control trial. The results showed an overall average net log reduction of 4.8 ±0.74, representing a greater than 99.99% reduction. For S epidermidis, less than 1.2% of the aerosolized bacteria were viable at 15 minutes. At 60 minutes, the average net log reduction was 5.95 ±0.34, equivalent to an almost 99.9999% reduction. For E herbicola, the average reduction at 15 minutes was >99.99%, and by 75 minutes, an average net log reduction of 5.36 ±0.37 was obtained. For the MS2 bacteriophage, in 15 minutes, on average, 99.999% was removed, and by 60 minutes, an average log reduction of 5.58 ±0.43 was observed. Testing with the Phi X174 bacteriophage produced an average net log reduction of 4.05 ±0.27 at 60 minutes. For both bacteriophages, reductions reached the limit of detection. For Bacillus subtilis, a 98.91% reduction in spores was found at 15 minutes, and by 90 minutes, a net log reduction of 4.23 ±0.31. was observed. Lastly, the 15-minute reduction for A niger was 99.71%, and by 60 minutes, an average net log reduction of 4.12 ±0.10 was found (Figure 3). At the 60-minute sampling point, the log reductions compared to the samples obtained ranged from a log 4 reduction (99.99% reduction) to a log 6 reduction (99.9999% reduction)41 (Figure 3).
Brondell Pro Sanitizing Air Purifier with AG+ Technology: This device was FDA-cleared as a medical device in January 2021 and is CARB-certified. It contains a pre-filter, proprietary HEPA filter, and nanocrystalline filter. The HEPA filter is described as antiviral; the nanocrystalline filter removes gases and odors; and a UV light is used inside the device to help sanitize the filter surfaces, the unit, and the air within it. A plasma generator generates negative ions that leave the device together with clean air and are designed to destroy micro-organisms in the room’s air. In one independent laboratory test using a test chamber, a 99.9% reduction in the H1N1 and H3N2 influenza viruses was found at 1 hour. In a second independent laboratory test, a 99.9% reduction in SARS-CoV-2 at 15 minutes was found. At the time of writing, no studies in healthcare settings were found.
Molekule Air Pro RX air purifier: This is an FDA-cleared medical device and CARB-certified. It contains a pre-filter and a photoelectrochemical oxidation (PECO) filter. The fibers of the PECO filter are coated with a nanocatalyst that is activated by UV light and produces hydroxyl radicals in the closed chamber to destroy micro-organisms trapped in the PECO-filter fibers. In an independent laboratory test using 4 filter samples per time point, the company’s PECO filters were evaluated for reductions in RNA virus MS2, a proxy virus for SARS-CoV-2.42 At 1 hour, a 99.95% reduction was observed, and at 24 hours, a 99.9994% reduction was observed.
Radic8: Radic8 devices were first developed in South Korea during the SARS outbreak and are used extensively there. This air purifier has 2 main phases.43 After the air is sucked in, it passes through a pre-filter, a HEPA filter, and an activated carbon filter. UV-C light and titanium dioxide are used to produce hydroxyl radicals—these remain in the closed chamber and kill micro-organisms there. Treated air is then released into the room. The device has a “single-pass” 99.9999% kill rate against viruses similar to SARS-CoV-2, meaning that this kill rate is achieved based on air going through the device once. This technology is proven in lab testing to kill SARS-CoV-2, bacteria, and fungi in aerosols. While not an FDA-cleared medical device, the Radic8 is currently marketed under the FDA’s Enforcement Policy for Sterilizers, Disinfectant Devices, and Air Purifiers During the Coronavirus Disease 2019 (COVID-19) Public Health Emergency.
CONCLUSION
Collectively, emerging and re-emerging diseases have resulted in a greater focus on infection control and, periodically, in changes to infection control guidance and practices. Of particular concern, we have now entered what is being referred to as the post-antibiotic era in which our ability to combat many infectious diseases is compromised by decreasing the availability of effective antibiotics. Our understanding of airborne transmission has also come into sharper focus, and during COVID-19, a layered approach has been recommended, including ventilation, as well as to consider HEPA filtration and UVGI. Numerous new types of adjunctive devices are on the market. When looking at newer devices, if considering one, a careful review and due diligence are needed to determine their efficacy, safety, and applicability.
REFERENCES
1. Gaynes R. The discovery of penicillin—new insights after more than 75 years of clinical use. Emerg Infect Dis. 2017;23(5):849–53. doi:10.3201/eid2305.161556
2. Centers for Disease Control and Prevention. Vaccine recommendations and guidelines of the ACIP. Updated July 16, 2013. Available at: https://www.cdc.gov/vaccines/hcp/acip-recs/index.html
3. Andre FE, Booy R, Bock HL, et al. Vaccination greatly reduces disease, disability, death and inequity worldwide. Bull World Health Organ. 2008;86(2):140–6. doi:10.2471/blt.07.040089
4. Centers for Disease Control and Prevention. Fact sheet: Multidrug-resistant tuberculosis (MDR TB). Updated May 4, 2016. Available
at: https://www.cdc.gov/tb/publications/
factsheets/drtb/mdrtb.htm
5. Phadke VK, Bednarczyk RA, Salmon DA, et al. Association between vaccine refusal and vaccine-preventable diseases in the United States: a review of measles and pertussis. doi:10.1001/jama.2016.1353
6. World Health Organization. Zoonoses. Updated July 29, 2020. Available at: https://www.who.int/news-room/fact-sheets/detail/zoonoses
7. Kohn WG, Collins AS, Cleveland JL, et al; Centers for Disease Control and Prevention (CDC). Guidelines for infection control in dental health-care settings–2003. MMWR Recomm Rep. 2003;52(RR-17):1-61.
8. Laheij AM, Kistler JO, Belibasakis GN, et al; European Oral Microbiology Workshop (EOMW) 2011. Healthcare-associated viral and bacterial infections in dentistry. J Oral Microbiol. 2012;4. doi:10.3402/jom.v4i0.17659
9. Centers for Disease Control and Prevention. Healthcare-associated infections (HAIs). Updated December 14, 2017. Available at: https://www.cdc.gov/winnablebattles/report/HAIs.html
10. Centers for Disease Control and Prevention. Winnable battles: healthcare-associated infections (HAIs). Updated December 14, 2017. Available at: https://www.cdc.gov/
winnablebattles/report/HAIs.html
11. United States Department of Labor: Occupational Safety and Health Administration. OSHA standards: 1910.1030-Bloodborne Pathogens. Section (d)(4)(iv). Available at: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1030
12. Hotez P. America and Europe’s new normal: the return of vaccine-preventable diseases. Pediatr Res. 2019;85(7):912-4. doi:10.1038/s41390-019-0354-3
13. Centers for Disease Control and Prevention: National Institute for Occupational Safety and Health. Environmental control for tuberculosis: basic upper-room ultraviolet germicidal irradiation guidelines for healthcare settings. March 2009. Available at: https://www.cdc.gov/niosh/docs/2009-105/default.html
14. Centers for Disease Control and Prevention. Candida auris. Updated July 22, 2021. Available at: https://www.cdc.gov/fungal/candida-auris/
15. Centers for Disease Control and Prevention. Legionella (Legionnaires’ disease and pontiac fever). Updated March 25, 2021. Available at: https://www.cdc.gov/legionella/about/causes-transmission.html
16. Tellier R, Li Y, Cowling BJ, et al. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19:101. doi:10.1186/s12879-019-3707-y
17. Fennelly KP. Particle sizes of infectious aerosols: implications for infection control. Lancet Respir Med. 2020;8(9):914-924. doi:10.1016/S2213-2600(20)30323-4
18. Yu IT, Li Y, Wong TW, et al. Evidence of airborne transmission of the severe acute respiratory syndrome virus. N Engl J Med. 2004;350(17):1731-9. doi:10.1056/NEJMoa032867
19. Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus (MRSA). Updated June 26, 2019. Available at: https://www.cdc.gov/mrsa/community/index.html
20. New York State Department of Health. Chickenpox. Varicella-zoster virus. Updated January 2014. Available at: https://www.health.ny.gov/diseases/communicable/chickenpox/fact_sheet.htm
21. Centers for Disease Control and Prevention. Pseudomonas aeruginosa in Healthcare Settings. Updated November 13, 2019. Available at: https://www.cdc.gov/hai/organisms/
pseudomonas.html
22. Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135(4):429-37. doi:10.14219/jada.archive.2004.0207
23. Xie X, Li Y, Chwang AT, et al. How far droplets can move in indoor environments—revisiting the Wells evaporation-falling curve. Indoor Air. 2007;17(3):211-25. doi:10.1111/j.1600-0668.2007.00469.x
24. Barron P. Centers for Disease Control and Prevention. Generation and behavior of airborne particles (aerosols). Available at: https://www.cdc.gov/niosh/topics/aerosols/pdfs/
Aerosol_101.pdf
25. Teska P. Pathogens underfoot can floor patients, health care workers. Infection Control Today. March 28, 2021. Available at: https://www.infectioncontroltoday.com/view/pathogens-underfoot-can-floor-patients-health-care-workers
26. Siegel JD, Rhinehart E, Jackson M, et al; Health Care Infection Control Practices Advisory Committee. 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Health Care Settings. Am J Infect Control. 2007;35(10 Suppl 2):S65-164. doi:10.1016/j.ajic.2007.10.007
27. Centers for Disease Control and Prevention. COVID-19: Guidance for dental settings. Updated December 4, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/dental-settings.html
28. American Academy of Oral and Maxillofacial Surgeons. Intraoral vs. extraoral suction devices. A review of the effectiveness of equipment on capturing aerosols. June 2, 2020. Available at: https://www.aaoms.org/docs/COVID-19/
Intraoral_vs_Extraoral_Suction_Devices.pdf
29. Centers for Disease Control and Prevention. Ventilation in buildings. Updated June 2, 2021. Available at: https://www.cdc.gov/coronavirus/2019-ncov/community/
ventilation.html
30. United States Department of Labor: Occupational Safety and Health Administration. Standard 1910.1000 – Air Contaminants. Updated March 26, 2015. Available at: https://www.osha.gov/laws-regs/regulations/standardnumber/1910/1910.1000
31. Environmental Protection Agency. Indoor Air Quality (IAQ): Ozone generators that are sold as air cleaners. Available at: https://www.epa.gov/indoor-air-quality-iaq/ozone-generators-are-sold-air-cleaners
32. The California Air Resources Board. About the California Air Resources Board. Available at: https://ww2.arb.ca.gov/about
33. California Air Resources Board. Hazardous ozone-generating air purifiers. Available at: https://ww2.arb.ca.gov/our-work/programs/air-cleaners-ozone-products/hazardous-ozone-generating-air-purifiers
34. Centers for Disease Control and Prevention. Infection control: Isolation precautions. Updated July 22, 2019. Available at: https://www.cdc.gov/infectioncontrol/guidelines/isolation/
35. The American Society of Heating, Refrigerating and Air-Conditioning Engineers. ASHRAE Epidemic Task Force: Dental Facilities. Updated April 21, 2021. Available at: https://www.ashrae.org/file%20library/technical%20resources/covid-19/ashrae-dental-c19-
guidance.pdf
36. National Collaborating Centre for Environmental Health. Indoor CO2 sensors for COVID-19 risk mitigation: current guidance and limitations. Published May 2021. Available at:
https://ncceh.ca/sites/default/files/FINAL%20-%20Using%20Indoor%20CO2%20Sensors%20for%20COVID%20MAY%2017%202021.pdf
37. Environmental Protection Agency. Indoor Air Quality (IAQ). What is a HEPA filter? Available at: https://www.epa.gov/indoor-air-quality-iaq/what-hepa-filter-1
38. The American Society of Heating, Refrigerating and Air-Conditioning Engineers. Filtration and disinfection FAQ. Available at: https://www.ashrae.org/technical-resources/filtration-and-disinfection-faq
39. ASHE. Air filtration. Updated 2014. Available at: https://www.ashe.org/compliance/ec_02_05_01/01/airfiltration
40. Environmental Protection Agency. Indoor Air Quality (IAQ). What is a HEPA filter? Available at: https://www.epa.gov/indoor-air-quality-iaq/what-hepa-filter-1
41. ActivePure Medical Dossier. Data on file.
42. Balarashti J, Conley Z. Kill kinetics of catalytic filters used in Molekule® Air Pro RX device against MS2 bacteriophage. Aerosol Research and Engineering Laboratories.
43. Radic8. VK 401. radic8.com/products/vk401.
ABOUT THE AUTHOR
Dr. Collins graduated as a general dentist from the University of Glasgow in Scotland and holds an MBA and an MA from Boston University. She is a published author and international speaker on topics including infection control and OSHA. She is a consultant; an editor for Dental World; a trainer; and a CE contributor, editor, and peer reviewer. She is the ADA representative to the Association for the Advancement of Medical Instrumentation (AAMI), Chicago Dental Society, the Organization for Safety, Asepsis and Prevention (OSAP), a participant in Standards working groups and a Fellow of the Pierre Fauchard Academy. She can be reached via email at drfionacollins@gmail.com.
Disclosure: Dr. Collins reports no disclosures.