INTRODUCTION
Local anesthetics (LAs) form the backbone of pain control techniques in dentistry. “Modern” dentistry began with the introduction of cocaine in 1885 and procaine (Novocain) in 1905, allowing dentists and surgeons to painlessly carry out procedures that previously had been impossible to do or were excruciatingly painful.
From the early 1900s until the mid-1940s, the “ester-type” LAs were used: Procaine, propoxycaine, and tetracaine were the most popular. In 1948, the first “amide-type” LA, lidocaine (Xylocaine), was introduced, quickly becoming the gold standard.
The 1960s and 1970s saw the introduction of other amides: mepivacaine (1960), prilocaine (1965), and bupivacaine (1972).
In dentistry, amide LAs have replaced the esters; indeed, in North America today, ester LAs are no longer marketed in dental cartridges.
Annually, more than 300 million cartridges are administered by dentists in the United States, another 30 million in Canada, and 1.96 billion worldwide.
With Dentistry Today celebrating its 40th anniversary this year, it was my thought to review the significant advances that have been made in the area of dental pain control over these past 40 years.
THE EARLY YEARS (1980 to 1999)
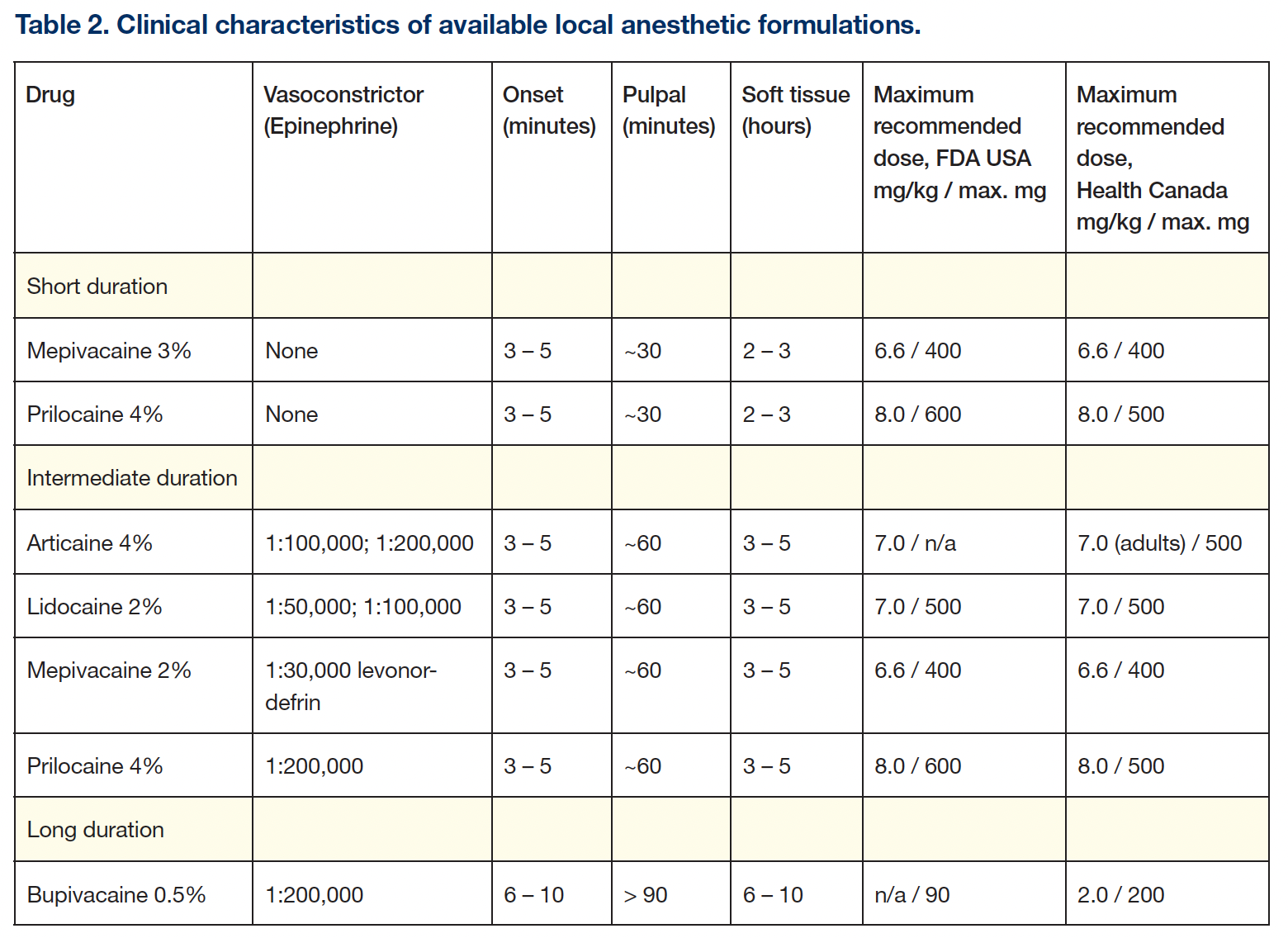
By the 1980s, in North America, 4 [more than 4 are mentioned] excellent LAs were available: mepivacaine and prilocaine, “plain” (no vasoconstrictor), for procedures of up to 30 minutes; mepivacaine, lidocaine, and prilocaine, with a vasoconstrictor, for procedures of up to 1 hour; and bupivacaine with epinephrine for post-surgical pain control.
With few exceptions (eg, infected mandibular molars), effective pain control for dental procedures was achievable. Still, consistently effective pain control with the traditional inferior alveolar nerve block (IANB, “mandibular block”) proved vexing to many dentists and dental hygienists, even for simple procedures. In response to the high failure rate associated with the IANB, alternative techniques of mandibular anesthesia were developed or reintroduced, including the Gow-Gates mandibular block, the Vazerina-Akinosiclosed-mouth mandibular block, the periodontal ligament injection (PDL), and intraosseous (IO).
Gow-Gates mandibular block: The Gow-Gates mandibular block technique had been introduced in 1973. Taught at many—but not all—dental schools, its rate of successful anesthesia, once learned, is considerably greater than the IANB.1
Another area of concern was palatal anesthesia. The density and sensitivity of palatal soft tissues and their adherence to bone made palatal injections one that most patients and, in many cases, their dentists reticent to receive or administer. The use of topical anesthetic, pressure, and slow injection served, to some degree, to minimize discomfort, but to put it simply, palatal injections hurt!
C-CLAD (computer-controlled local anesthetic delivery): In 1997, Dr. Mark Hochman published the first paper describing the use of a local anesthetic delivery system employing a computer to control the rate and pressure of LA delivery.2 That device, the Wand (Milestone Scientific), represented an evolution in LA delivery, moving away from the traditional metal aspirating dental syringe.
C-CLAD devices permit the precise administration of LA at a steady rate and pressure, providing a more comfortable injection experience for the patient. Though designed for those injections deemed more uncomfortable, palatal, PDL- C-CLAD devices may be employed for any and all intraoral injections.
Hochman compared palatal injections with a traditional syringe and the Wand and found 96% of subjects preferred the C-CLAD instrument.2 Visual analog scale (VAS) measurements were significantly lower for C-CLAD vs traditional syringes.
Subsequent research with C-CLAD led to the development of 2 new LA techniques: anterior, middle superior alveolar (AMSA) nerve block and palatal-approach anterior superior alveolar (P-ASA) nerve block.
Since the introduction of the Wand in 1997, other C-CLAD systems have been introduced, including the Wand STA (Milestone Scientific) and Dentapen (Septodont).
Published research trials have demonstrated the utility of C-CLAD in all patients,3 especially in the pediatric population.
RECENT DEVELOPMENTS (2000 to 2021)
From 2000 to the present, the years have seen the development and introduction of a number of valuable adjuncts to pain control.
Articaine HCl (2000): In 1999, in the United States, 3 excellent, highly effective, and safe LAs were available (lidocaine, mepivacaine, and prilocaine) that, with the addition of a vasoconstrictor, provided pulpal anesthesia for approximately 1 hour. Bupivacaine, a long-duration LA, is primarily used for post-surgical pain control.
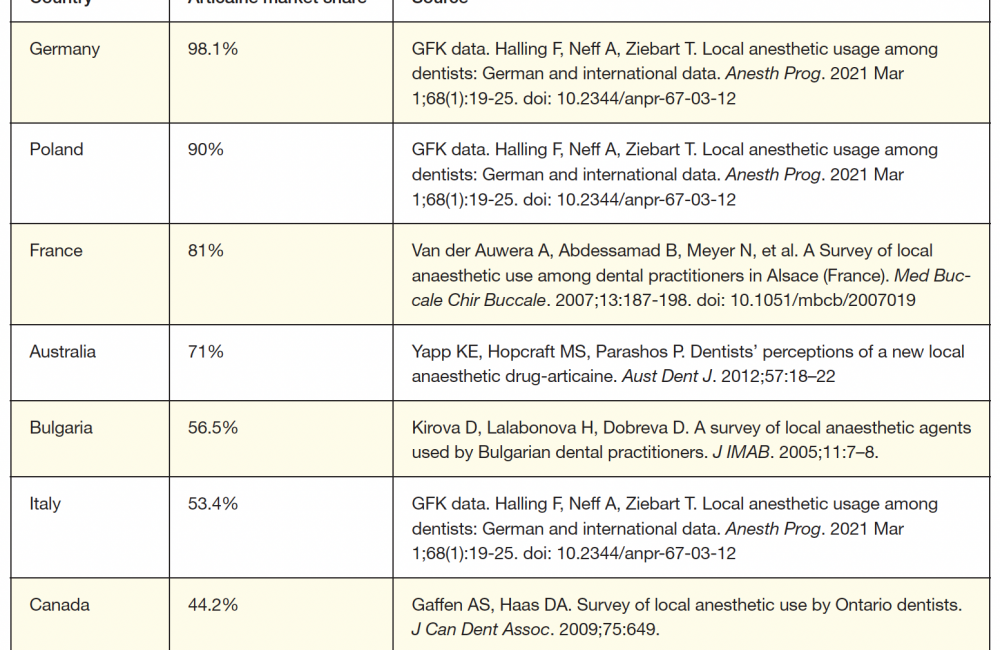
A fifth LA—articaine—was available worldwide, but not yet in the United States in 2000. Synthesized in Germany in 1969, articaine was introduced into clinical practice there in 1976, representing the first, and still only, LA designed for dentistry. Its clinical characteristics (eg, onset, duration) are similar to those of the other intermediate-duration LAs. Marketed as a 4% solution with epinephrine in 1:100,000 and 1:200,000 concentrations, articaine has become the preferred LA in most countries (Table 1).
In June 2000, the Food and Drug Administration (FDA) approved articaine for use in the United States in patients aged 4 and up, and it quickly become popular—but why?
Anecdotal reports from dentists beginning to use articaine stated that: “it works faster,” “it works better,” “I don’t miss as often,” and “hard-to-numb patients are easier to numb.” Of course, anecdotal reports are not science.
In phase 3, randomized, controlled, double-blinded trials (RCT) in the United States, it was concluded that articaine was “as safe and as effective as lidocaine,”4 the drug to which it was compared.
Articaine does possess chemical and clinical characteristics that make it, in this author’s opinion, the preferred LA in many situations. These characteristics, articaine’s advantages, and the “controversy” regarding an increased risk of paresthesia following IANB are discussed in a subsequent section.
Phentolamine mesylate—the LA “off switch” (2008): The addition of a vasoconstrictor confers the following benefits on the LA: (1) increased depth, (2) increased duration, and (3) increased safety (by decreasing the LA blood level). Associated with these benefits is an increase in the duration of soft-tissue anesthesia to 3 to 5 hours. Following surgical procedures, residual soft-tissue anesthesia (RSTA) is appreciated; however, most dental procedures do not require post-treatment pain control. In some instances, self-inflicted soft-tissue injury (SISTI) may result from chewing or biting on the still-numb lip or tongue. Though most common in younger children, the incidence of SISTI is significant in special needs and geriatric patients. Additionally, many patients would appreciate having soft-tissue anesthesia “go away” more quickly so that they could return to their usual lifestyles—eating, social gatherings, business meetings, etc, where RSTA can be an embarrassment or lead to soft-tissue injury.
Phentolamine mesylate (PM), an alpha-adrenergic antagonist (eg, vasodilator), available in dental cartridges and injected at the end of the “painful” part of the dental treatment (eg, drilling or root planing) into the same location that the LA was deposited earlier, significantly decreases the duration of RSTA.
In a study by Malamed et al,5[ ref 5 is Malamed alone, wrong ref?] complete loss of anesthesia in the upper lip at 30 minutes following PM administration occurred in 26.7% vs 1.7% of controls; at 60 minutes, it was 59.2% vs 11.7%. In the lower lip, at 30 minutes, 17.2% of PM subjects had complete loss of anesthesia vs 0.8% of controls, and at 60 minutes, it was 41% PM vs 7.4% controls.
PM (OraVerse [Septodont]) has been approved by the FDA for administration to patients age 3 and up.
Buffered local anesthetics—The LA “on switch” (2010): Vasoconstrictor-containing LAs are highly acidic, with a pH ranging from 3.0 to 4.0. Injection of this acidic solution is associated with a “stinging” or “burning” sensation and a slower onset of anesthesia (compared to plain LAs). Addition of a defined volume of 8.4% sodium bicarbonate to a dental cartridge—raising its pH to approximately 7.35 to 7.4—provides a more comfortable injection experience for the patient. Additional benefits of buffering include (1) more rapid onset of pulpal anesthesia,6 more profound anesthesia,7 and decreased post-injection soreness.
In an RCT comparing lidocaine 2%, epinephrine 1:100,000, buffered to a pH of 7.35, with unbuffered (pH 3.5) for IANB, the onset of pulpal anesthesia was 1 minute, 51 seconds (buffered), vs 6 minutes, 37 seconds unbuffered.6
The methods of buffering are (1) DIY (do it yourself) and (2) onset (the original dental buffering system). DIY is more cost-effective but runs the risk of the resulting pH of LA solution being higher than desired (7.35 to 7.4). With an elevated pH (> 7.5), edema (swollen lip and/or cheek) at the injection site is a common occurrence. With the dental buffering system, the pH consistently ranges from 7.35 to 7.4.
In a 2019 Journal of the American Dental Assosiation paper,7 buffered LAs were reported to be more effective than nonbuffered LAs both by mandibular and maxillary injection in pulpally involved teeth. Buffered LAs had a 2.29 greater likelihood of achieving successful anesthesia.
Though not all assessments of buffering have produced similar results, it is this author’s recommendation that all LA injections be buffered.
ARTICAINE: AN IN-DEPTH LOOK
As mentioned earlier and illustrated in Table 1, articaine is a popular LA. Worldwide, 600 million articaine cartridges are administered annually, exceeded only by lidocaine’s 1 billion cartridges.8
There are 2 distinct pharmacokinetic and chemical characteristics that make articaine popular: (1) more rapid elimination from the cardiovascular system (elimination half-life) and (2) lipid solubility.
More rapid elimination leads to increased safety, and it’s being a preferential drug, in this author’s opinion, in patients who are pregnant, nursing, geriatric, and pediatric, while being more lipid-soluble leads to more rapid onset and greater depth of anesthesia. Additionally, articaine’s greater lipid solubility enables it to be successful using injection techniques that have been uniformly unsuccessful with other LAs.
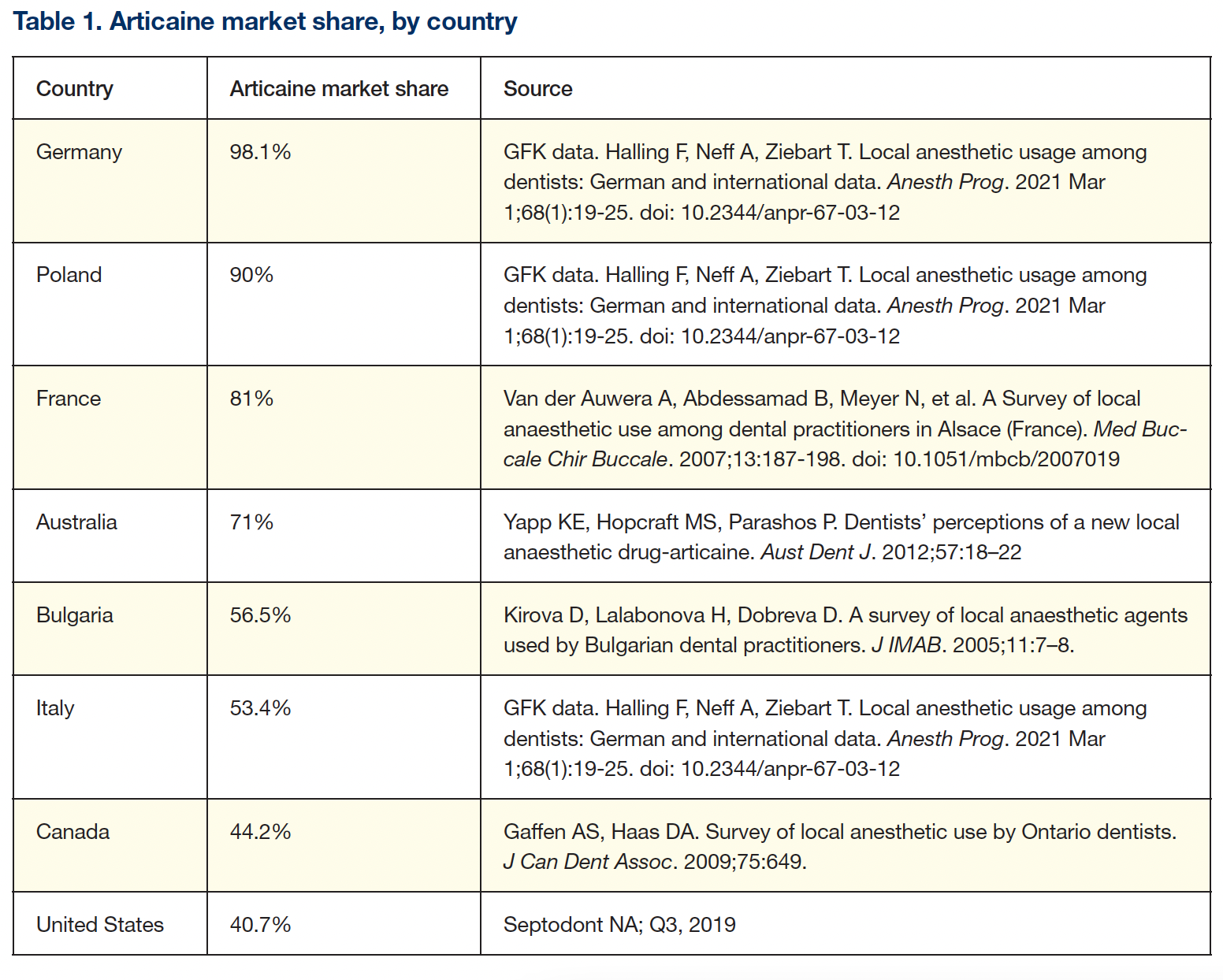
Elimination half-life: Classified as an amide-LA, articaine is actually a hybrid molecule, possessing both amide and ester bonds (Figure 1).
Anesthesia “stops” when LA diffuses out of the nerve and into the cardiovascular system (CVS): to capillaries, veins, the heart, and then arteries, circulating the LA throughout the body. This process is called “redistribution.” The LA—not yet metabolized—is an active drug upon entering the CVS. A measurable blood (plasma) level of the LA occurs.
The significance of LA blood level is LAST (local anesthetic systemic toxicity), also known as overdose or toxic reaction. An overly high plasma level of any drug in its target organ(s) produces overdose. Target organs for LAs are the brain (central nervous system) and myocardium. The higher the plasma level, the greater the overdose risk. Plasma levels of LAs decrease primarily through metabolism (biotransformation, detoxification).
Amide LAs undergo metabolism almost entirely in the liver (hepatic microsomal enzymes), whereas ester LAs are metabolized in the blood by plasma esterases.
The elimination half-life of a drug—the time for its plasma level to decrease by 50%—for most amide LAs is approximately 90 minutes (hepatic metabolism). Esters have a considerably shorter half-life (plasma esterase metabolism). For procaine, it’s 6 minutes; for tetracaine, it’s 20 minutes.
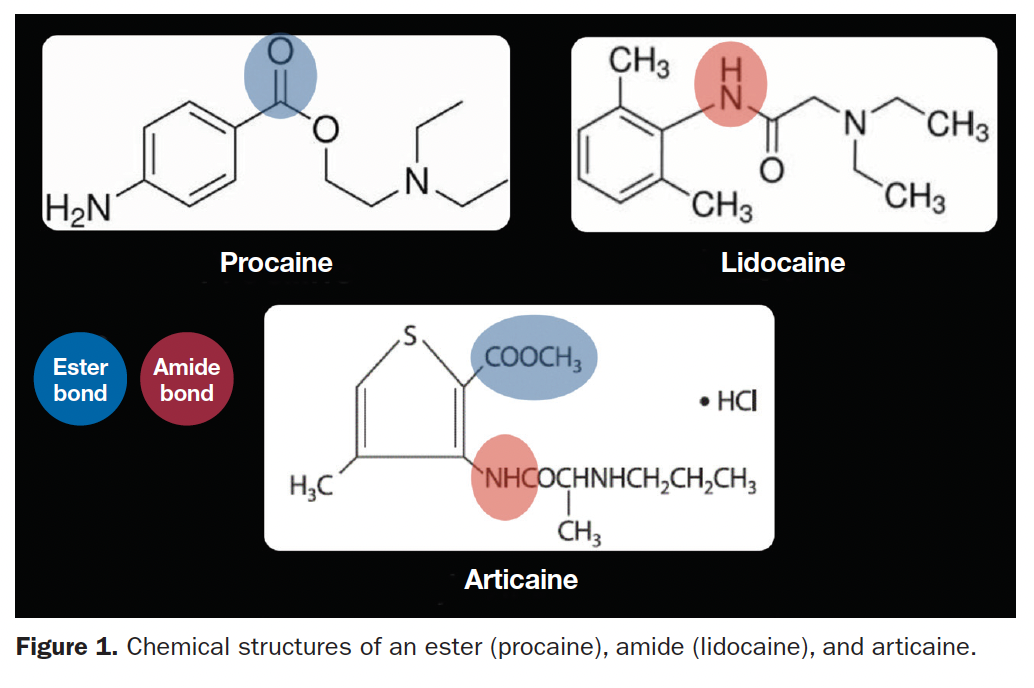
Between 90% to 95% of articaine metabolism is plasma esterase. In adults, articaine’s elimination half-life is 20 to 27 minutes; for geriatric patients, it’s 27 minutes; in pediatric patients, it’s 18.5 to 23.6 minutes9 (Figure 2).
LAST resulting from “proper” administration (aspirate 2 times, administer slowly) of an overly large LA dose is considerably less likely with articaine than other amide LAs. One must always remember that one cartridge of any LA administered rapidly intravascularly will produce LAST.
Lipid solubility: For LA to diffuse through a nerve membrane, it must be lipid-soluble. Lipid solubility is conferred through an aromatic ring—part of the drug’s chemical configuration. Most LAs possess a benzene ring (Figure 1). Articaine possesses a more lipid-soluble thiophene ring.
The anecdotal significance of increased lipid solubility is “I don’t miss as often,” “hard-to-numb patients are easier to numb with articaine,” and “it works better.” Randomized, controlled clinical trials have proven these claims true.
Articaine by Mandibular Buccal Infiltration (BI) in Adults
Research has demonstrated the efficacy of articaine mandibular BI in adults. Robertson et al10 infiltrated 1.8 mL of either lidocaine or articaine by the first mandibular molar as the primary injection for pain control. Pulpal anesthesia for lidocaine was 45% second molar, 57% first molar, 67% second premolar, and 61% first premolar. Articaine results were 75%, 87%, 92%, and 86%.[“, respectively”?] Lidocaine onset times ranged from 6.3 minutes (first premolar) to 11.1 minutes (second molar); onset for articaine ranged from 4.2 to 4.7 minutes.[“, respectively”?]
Buccal infiltration of articaine as a supplement to IANB significantly increases IANB success rates. Kanaa et al11 showed a 55.6% success rate for IANB on the first molar with lidocaine and epinephrine. When articaine BI was administered, the success rate went to 91.7%, not showing any decrease by the end of the 45-minute study.
It is this author’s recommendation that all IANBs, regardless of the LA used, be followed by BI of articaine (0.9 mL) at the tooth being treated.
Articaine by Maxillary Buccal Infiltration for Palatal Soft-Tissue Anesthesia in Adults
Palatal injections are dreaded by dental patients and by most dentists and hygienists. Yet palatal anesthesia is frequently necessary for dental procedures.
Clinical trials comparing the efficacy of articaine and lidocaine in providing palatal soft-tissue anesthesia following maxillary BI have demonstrated articaine’s superiority. Gholami et al12 repeated the infiltration of 0.6 mL of LA at 2-minute intervals and evaluated palatal anesthesia. At 4 minutes (1.2 mL), 65.4% of articaine patients and 0.65% of lidocaine patients had palatal anesthesia. At 6 minutes (1.8 mL), values were 82.7% vs 1.3%. Results were similar in the anterior, premolar, and posterior maxillary regions.
Though not all studies demonstrated this same success rate, it is this author’s recommendation that BI of 1.2 or 1.8 mL articaine be administered, with palatal anesthesia evaluated after 4 (1.2 mL) and 6 minutes (1.8 mL). If palatal anesthesia proves less than ideal at 6 minutes, administer a palatal infiltration. Some degree of palatal anesthesia should be present, making the palatal infiltration more comfortable for the patient.
Controversy: Does Articaine, Administered By IANB, Have a Greater Risk of Paresthesia Than Other LAs?
In 1995, Haas and Lennon13 reported on the incidence of paresthesia following LA administration in Ontario, Canada. Most reports involved the lingual nerve (70.6%). The calculated incidence of paresthesia following LA injection (all drugs) was 1 in 785,000; for articaine by IANB, the incidence was 1 in 440,000. In a subsequent paper by Garisto et al,[is this not a ref?] the calculated risk of paresthesia for articaine in the United States was 1 in 4.1 million injections, with an overall risk (all LAs) of 1 in 13.8 million. Other published case reports of paresthesia following articaine[injections?] recommended that articaine not be administered by IANB.
It is estimated that in a 20-year practice career, approximately 30,000 IANBs will be administered.14 Pogrel and Thamby,14 in a paper published prior to articaine’s arrival in the United States, estimated the risk of permanent nerve damage following IANB at 1 in 26,762 injections, stating: “it is reasonable to suggest that during a career, each dentist may encounter at least one patient with an IANB resulting in permanent nerve involvement. The mechanisms are unknown, and there is no known prevention or treatment.”
Recent research on LA neurotoxicity using human neuroblastoma cells (precursor nerve cells) has shown that (1) all LAs are neurotoxins and (2) amongst dental LAs, articaine and ropivacaine demonstrate the “lowest toxicity”; mepivacaine, prilocaine, and lidocaine were “medium toxicity”; and bupivacaine had the “highest toxicity.”15
There is no scientific evidence demonstrating that articaine is more neurotoxic than other LAs. Almost all publications present case reports or opinion papers—the lowest form of scientific evidence. Meta-analyses and systematic reviews—the most reliable science—have confirmed that articaine is a “better” LA and no more neurotoxic than other LAs.16
Addressing the controversy regarding articaine paresthesia, Dr. Gordon Christensen, a well-respected dental educator and clinical researcher, stated: “Studies have not shown that to be true. The controversy is unfounded.”17
FORTY YEARS LATER: RECOMMENDATIONS
As we celebrate 40 years of Dentistry Today and complete our review of the evolution of dental pain control, I leave you with several recommendations:
1. Articaine has been clearly demonstrated to be more effective than other dental LAs.
- Routinely administer 0.9 mL by BI at the apex of a mandibular molar being treated following IANB with any LA.
- Articaine maxillary BI, 1.2 to 1.8 mL (waiting 4 to 6 minutes), provides palatal soft-tissue anesthesia in a large majority of adult patients.
2. Buffer LA injections. Buffering LA solutions provides faster onset of more profound anesthesia and is more comfortable on injection and less uncomfortable for the patient postoperatively. Though all injections—maxillary and mandibular—can, and should, be buffered, it is in the mandible where the greatest benefit is noted.
3. C-CLAD: The ability to provide a painless injection is the No. 1 criteria patients use when evaluating their doctors. C-CLAD devices enable significantly more comfortable injections to be administered.
4. PM: Post-treatment RSTA is, at a minimum, uncomfortable and undesirable and potentially a source of trauma. The ability to significantly decrease the duration of soft-tissue anesthesia will be desirable for many patients.
Here is one final note:
In 1981, only 10 states permitted the administration of LAs by dental hygienists. Today, LA administration is permitted in 45 states.18 Only 5 to go.
REFERENCES
1. Gow-Gates GA. Mandibular conduction anesthesia: a new technique using extraoral landmarks. Oral Surg Oral Med Oral Pathol. 1973;36(3):321–8. doi:10.1016/0030-4220(73)90208-9
2. Hochman M, Chiarello D, Hochman CB, et al. Computerized local anesthetic delivery vs. traditional syringe technique. Subjective pain response. N Y State Dent J. 1997 Aug-Sep;63(7):24–9.
3. Flisfisch S, Woelber JP, Walther W. Patient evaluations after local anesthesia with a computer-assisted method and a conventional syringe before and after reflection time: A prospective randomized controlled trial. Heliyon. 2021;7(2):e06012. doi:10.1016/j.heliyon.2021.e06012
4. Malamed SF, Gagnon S, Leblanc D. Efficacy of articaine: a new amide local anesthetic. J Am Dent Assoc. 2000;131(5):635–42. doi:10.14219/jada.archive.2000.0237
5. Malamed SF. Local anesthesia reversal. Dent Today. 2010;29(3):65–6, 68, 71-2 passim; quiz 74.
6. Malamed SF, Tavana S, Falkel M. Faster onset and more comfortable injection with alkalinized 2% lidocaine with epinephrine 1:100,000. Compend Contin Educ Dent. 2013;34 Spec No 1:10-20.
7. Kattan S, Lee SM, Hersh EV, et al. Do buffered local anesthetics provide more successful anesthesia than nonbuffered solutions in patients with pulpally involved teeth requiring dental therapy?: A systematic review. J Am Dent Assoc. 2019;150(3):165-177. doi:10.1016/j.adaj.2018.11.007
8. Communication with Septodont Holding. Saint Maur des Fosses Cedex, France. May 2017.
9. Cazaubon Y, Mauprivez C, Feliu C, et al. Population pharmacokinetics of articaine with 1:200,000 epinephrine during third molar surgery and simulation of high-dose regimens. Eur J Pharm Sci. 2018;114:38-45. doi: 10.1016/j.ejps.2017.11.027
10. Robertson D, Nusstein J, Reader A, et al. The anesthetic efficacy of articaine in buccal infiltration of mandibular posterior teeth. J Am Dent Assoc. 2007;138(8):1104–12. doi:10.14219/jada.archive.2007.0324
11. Kanaa MD, Whitworth JM, Corbett IP, et al. Articaine buccal infiltration enhances the effectiveness of lidocaine inferior alveolar nerve block. Int Endod J. 2009;42(3):238–46. doi:10.1111/j.1365-2591.2008.01507.x
12. Gholami M, Banihashemrad A, Mohammadzadeh A, et al. The Efficacy of 4% Articaine Versus 2% Lidocaine in Inducing Palatal Anesthesia for Tooth Extraction in Different Maxillary Regions. J Oral Maxillofac Surg. 2021;S0278-2391(21)00195-6. doi:10.1016/j.joms.2021.02.019
13. Haas DA, Lennon D. A 21 year retrospective study of reports of paresthesia following local anesthetic administration. J Can Dent Assoc. 1995;61(4):319–20, 323–6, 329–30.
14. Pogrel MA, Thamby S. Permanent nerve involvement resulting from inferior alveolar nerve blocks. J Am Dent Assoc. 2000;131(7):901–7. doi:10.14219/jada.archive.2000.0308.
15. Malet A, Faure MO, Deletage N, et al. The comparative cytotoxic effects of different local anesthetics on a human neuroblastoma cell line. Anesth Analg. 2015;120(3):589–96. doi:10.1213/ANE.0000000000000562.
16. Stirrup P, Crean S. Does articaine, rather than lidocaine, increase the risk of nerve damage when administered for inferior alveolar nerve blocks in patients undergoing local anaesthesia for dental treatment? A mini systematic review of the literature. Br Dent J. 2019;226(3):213-223. doi: 10.1038/sj.bdj.2019.98.
17. Christensen GJ. Observations on Current Controversies in Dentistry. Dent Today. 2015;34(11):100, 102, 104-5.
18. American Dental Hygienists’ Association. States that permit dental hygienists to administer local anesthetics. https://www.adha.org/resources-docs/7521_Local_Anesthesia_by_State.pdf. Accessed 15 May 2021.
ABOUT THE AUTHOR
Dr. Malamed graduated from the New York University College of Dentistry in 1969 and completed a dental internship and residency in anesthesiology at Montefiore Hospital and Medical Center in the Bronx, NY, before serving for 2 years in the US Army Dental Corps. In 1973, Dr. Malamed joined the faculty of the University of Southern California School of Dentistry in Los Angeles where today he is a professor of anesthesia and medicine. Dr. Malamed is a Diplomate of the American Dental Board of Anesthesiology as well as a recipient of the Heidebrink Award (1996) from the American Dental Society of Anesthesiology and the Horace Wells Award (1997) from the International Federation of Dental Anesthesia Societies. He has authored more than 120 scientific papers and 16 chapters in various medical and dental journals and textbooks in the areas of physical evaluation, emergency medicine, local anesthesia, sedation, and general anesthesia. He authored the textbooks Handbook of Local Anesthesia (fifth edition), Emergency Medicine in Dentistry (sixth edition), and Sedation: A Guide to Patient Management (fourth edition). Dr. Malamed speaks and writes extensively on the subjects of emergency medicine, physical evaluation, local anesthesia, sedation, and general anesthesia. He can be reached at malamed@usc.edu.
Disclosure: Dr. Malamed is a consultant to OnPharma
RELATED ARTICLES
Allergy and Toxic Reactions to Local Anesthetics
Alternatives for Topical Anesthesia
A Review of Paresthesia in Association with Administration of Local Anesthesia