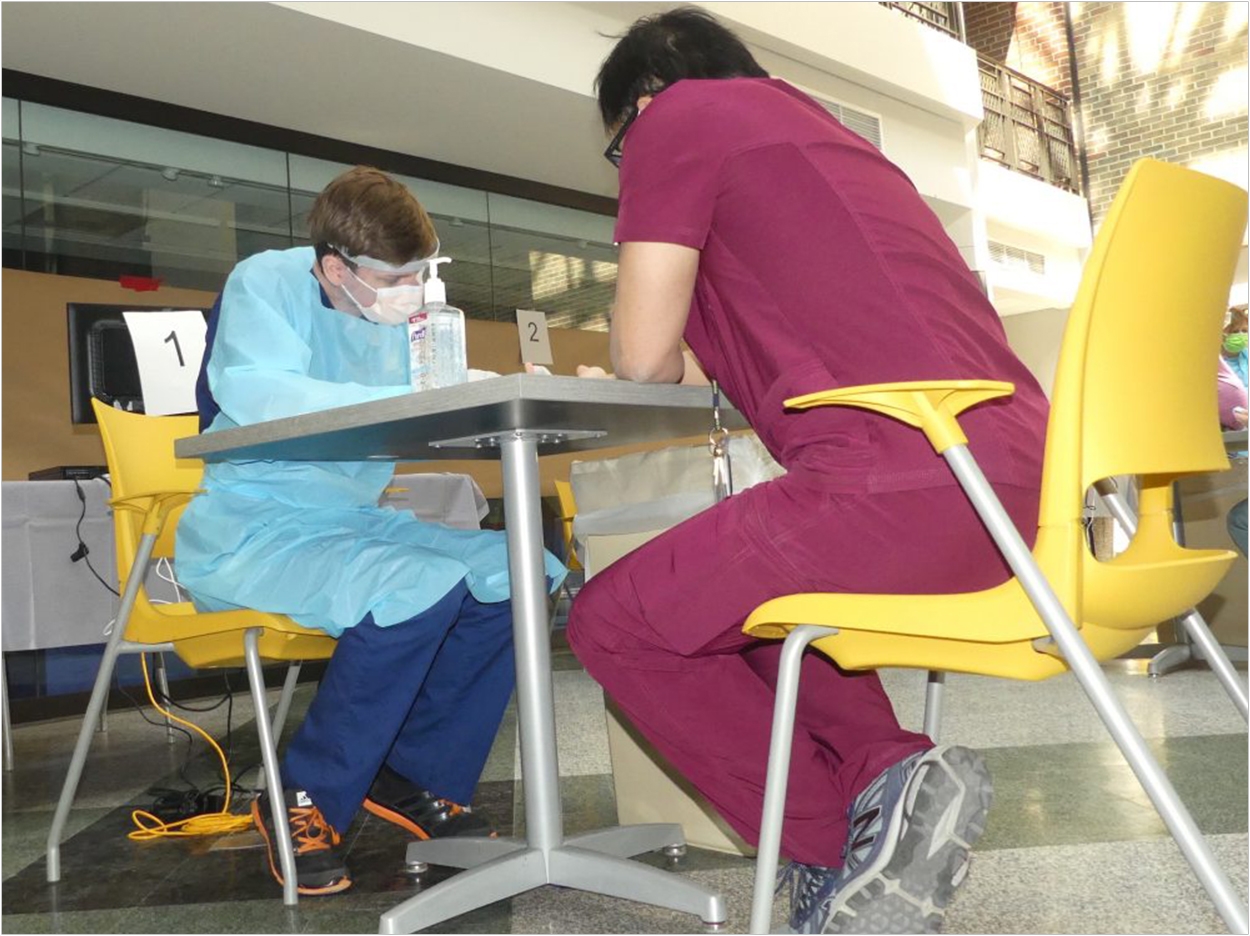
The University of Michigan School of Dentistry has joined a national effort to increase antibody testing related to the COVID-19 pandemic.
In April, the school began testing local frontline healthcare workers and more recently expanded to a wider community including all of its faculty, staff, and students as well as first responders and essential workers.
Only people without symptoms of the virus are being tested after they complete a health history survey. Those being tested have the option to participate in a research survey to determine how many have antibodies, factors related to infection with the SARS CoV-2 virus, and perceptions of the COVID-19 pandemic.
Participants signed up online, took the health history survey, and scheduled appointments. The last day of testing is Friday, June 19, and all appointments are filled through that date.
The project is being coordinated by several faculty members and staff who spent three weeks preparing protocols, procuring supplies, obtaining approval to conduct the research survey, and training those who are administering the tests.
The blood test detects the presence of antibodies created by the immune system’s response to the coronavirus. The tests can indicate whether a person has been infected with the virus, but they do not detect the actual virus.
Questions remain about whether people who have had the virus are immune from contracting it a second time, but the Centers for Disease Control and Prevention and other public health officials say widespread antibody testing is important. It will help answer the immunity question and document how many people across the country have been infected, which in turn can help in developing a process for reopening society, the school said.
In announcing the test during an online town hall meeting for the dental school, dean Laurie McCauley emphasized that the type of assay being used by the dental school has been testing by Michigan Medicine’s Department of Pathology.
“This test we are using has been validated here on our campus, with known positives and negatives,” McCauley said.
That’s important because national attention in recent weeks has questioned the reliability of antibody tests, many of which were quickly developed as the pandemic grew and haven’t been approved by the Food and Drug Administration, the school said, adding that some have given false positives and negatives.
Dr. Robert Eber, a clinical professor in the Department of Periodontics and Oral Medicine and the director of clinical research, is leading the team of faculty members who are working in conjunction with several other universities around the country. They are partnering with Henry Schein.
Over the last several weeks of planning, regular conference calls have been held with Henry Schein representatives and other dental school collaborators at New York University, Rutgers, Temple, Penn, and the University of California at San Francisco.
The antibody tests look for proteins in the blood called immunoglobulin M (IgM) and immunoglobulin G (IgG), which the body uses to identify infection by bacteria, viruses, and other foreign substances. Once antibodies identify the threat, the body then attacks the invader.
The presence of those antibodies, and which type, can indicate the timeline for a person’s infection. For example, IgM antibodies typically arrive within days of the infection, and IgG appears six to 10 days after the infection.
The procedure being used at the dental school requires a finger prick to obtain a few drops of blood from each participant. Tests are performed for IgM and IgG, each on a separate cassette. A drop of blood is placed in a well in each of the cassettes, and then a liquid reagent is added in a separate well to activate the test.
After about 15 minutes, color-coded lines indicate whether the test was run properly and whether the individual is positive or negative for IgM and IgG antibodies.
Related Articles
Program Reprocesses N95 Respirators for Future Use
BC Dental Practice Renovates to Meet Isolation Room Standard
App Determines Likelihood of Severe COVID-19 Cases


