|
UNDERSTANDING THE ETIOLOGY OF DENTIN HYPERSENSITIVITY
The lifestyle behaviors and oral self-care habits of today’s dental patients pose a number of challenges that may contribute to increased dentin hypersensitivity. It is through greater awareness and knowledge of the risk factors that we may more effectively manage dentin hypersensitivity for the dental patient. In the early 1980s, dentin hypersensitivity was described as an enigma because it was frequently encountered yet poorly understood.1 Before we begin discussing the etiology of dentin hypersensitivity, risk factors, and prevention strategies, it is important to fully understand the definition of dentin hypersensitivity.
Hypersensitivity may be defined as a short or transient sharp pain of a rapid onset that arises from exposed dentin and cannot be ascribed to any other dental defect or pathology.2 The pain is in response to a nonnoxious stimulus, or in other words, one that would not under normal circumstances create pain or discomfort.3 The cessation of pain is directly related to the removal of a stimulus. The definition provides a clinical descriptor of the condition and identifies dentin hypersensitivity as a distinct clinical entity.
The hypersensitivity of dentin is based on Brännström’s hydrodynamic theory that stimuli create a pressure change or disturbance within the fluid that fills the dentinal tubules. The movement of the fluid in the open tubules is then transmitted to the A-delta nerve fibres. Heat, cold, air, and pressure can cause this rapid movement of fluid in open dentin tubules. Cold stimuli will cause the fluid in the tubules to contract while heat stimuli will expand the fluid, both of which will cause a notable pressure change within the tubules.4,5
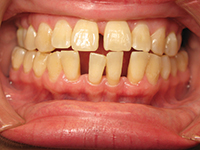
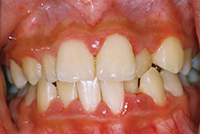
The phenomenon of dentin hypersensitivity is characterized predominantly by erosion, which both exposes dentin and more importantly initiates the lesions. Dentin hypersensitivity then occurs when dentin becomes exposed and tubules are open at the dentin surface. Gingival recession is the primary way dentin is exposed in the cervical region of the tooth. Once the root is exposed, the protective layer of cementum is easily removed, resulting in open dentin tubules. Other causes of the typically short and sharp pain may include caries, chipped teeth, fractured or faulty restorations, specific restorative materials and cracked tooth syndrome. It is important to rule out any other possible etiology before proceeding with specific management of dentin hypersensitivity.
The profile of the patient who suffers from dentin hypersensitivity is varied with the majority of sufferers between the ages of 20 to 50 years, peak sensitivity appears to be in the age group of 30 to 40 years. The decline after the fifth decade of life may be due to the development of secondary or sclerotic dentin.6 There also appears to be gender specificity with females significantly more likely to experience sensitivity than males.1 Published studies show extreme variation in prevalence as well as in process of identification of dentin hypersensitivity. It was also noted that dentin hypersensitivity is more frequently encountered in patients with periodontitis.
|
CLINICAL FEATURES OF DENTIN HYPERSENSITIVITY
The histology of dentin helps us to further understand dentin hypersensitivity. Dentin is naturally sensitive owing to its close structural and functional relationship with the dental pulp. Normally the dentin is well protected so sensitivity does not present an issue. It is a calcified tissue of the body usually covered by enamel on the crown and cementum on the root surface. By weight, 70% of dentin consists of hydroxyapatite, with 20% being organic material and 10% water.7 Dentin consists of microscopic channels called dentinal tubules which radiate outward through the dentin from the pulp to the exterior cementum or enamel border. As a result dentin has a degree of permeability which can increase the sensation of pain. Dentin is thought to be covered by a smear layer, consisting of a combination of both inorganic and organic elements, which occludes the dentinal tubule orifices forming a smear plug or natural “bandage” that blocks stimuli. Conversely, removal of these occluding materials can also frequently occur as a result of physical or chemical agents that open the dentinal tubules.
If the hydrodynamic theory is to be accepted as the mechanism involved for inducing dentin hypersensitivity, then lesions must have open dentin tubules at the surface. Through the use of scanning electron microscopy as well as dye penetration, studies have demonstrated the presence of a greater number (up to 8x) and wider tubules with average diameter being 2x greater on hypersensitive dentin compared to nonsensitive dentin. Depending on the depth of the sample, about 30,000 tubules can be found in a 1-mm2 cross-section of dentin.8
There is also evidence that tubule numbers and diameters increase from the outer dentin toward the pulp. The difference in tubule diameter is an important variable as the fluid flow is proportional to the fourth power of the radius, resulting in a 16-fold increase in fluid flow. Being it is the fluid flow that excites the pulpal receptors, there is conclusive evidence that tubule diameter plays an important part and that greater fluid flow plays a predominant role in dictating the intensity of hypersensitivity experienced.9
The majority of studies report that gingival recession occurs in descending order of canines and premolars followed by incisors and second premolars and finally molars with the majority of sites being buccal and cervical. In comparison, the majority of studies show a similar preference of distribution for gingival recession and both conditions have been shown to be more common on the left than on the right sides of the mouth and also possess an inverse relationship with plaque scores. This provides some interesting conclusive evidence to support that the majority of the population are right-handed and most likely exhibit a heavier brushing force on the opposite side of the mouth.10 The other factor to note is that there is an inverse relationship with the plaque score again supporting that the brushing force may be heavier on the opposing side.
|
EVALUATION STRATEGIES
Both chronic and acute pain related to dentin hypersensitivity can be particularly challenging for our dental hygiene patient. Acute pain often causes anxiety and may lead to avoidance of dental treatment. An appreciation for the impact of the pain on a patient’s quality of life and a more thorough understanding of hypersensitivity may lead clinicians to be more sympathetic. Often our patients will not schedule an appointment specifically to address their sensitivity. It is unlikely that the topic will even be discussed without some form of prompting by the dental hygienist. It is our responsibility to initiate the discussion and also to evaluate the level of sensitivity the patient is experiencing. Dental hygienists play an important proactive role in first identifying and then the subsequently managing dentin hypersensitivity both chairside and also with self-care recommendations.
There are a number of questions we may employ with our dental hygiene patient in order to obtain further information regarding dentin hypersensitivity. The following questions are suggested to initiate the discussion of dentin hypersensitivity also aiding to evaluate the intensity of pain:3
1. Which tooth or teeth is/are sensitive and on which aspect?
2. On a scale from one to 10, how much does it hurt, with 10 being the most painful?
3. How long does the pain last?
4. Can the pain be characterized as sharp, dull, shooting, throbbing, persistent, constant, pressure, burning, intermittent?
5. Does it hurt when you bite down (pressure)?
6. Does the discomfort linger or stop immediately after a stimulus such as cold water is removed?
7. On a scale from one to 10, how much does the pain impact your daily life?
8. Is the pain stimulated by certain foods: Sweet? Sour? Acidic?
9. Does sensitivity result from hot or cold food or beverages?
10. Does discomfort stop immediately upon removal of the painful stimuli, such as cold food or beverage?
11. How effectively are you managing the stress in your life?
There are a number of ways to evaluate the degree of hypersensitivity. Using a verbal rating scale and the use of a short, intermittent “air blast” to quantify the subjective level of pain as follows:3
0—No discomfort/pain; no discomfort/pain but aware of stimulus
1—Mild discomfort/pain—described as mild discomfort during but not following the air blast
2—Marked discomfort/pain—described as definitive discomfort during the air blast
3—Marked discomfort/pain lasting more than 10 seconds following exposure to air.
There are a number of predisposing factors that individually or collectively place the patient at risk for dentin hypersensitivity. These include gingival recession, tooth wear, lifestyle behaviors, oral self-care habits, and the pH of the oral environment which may be related to dietary as well as xerostomic conditions.
The assessment and evaluative phase of the dental hygiene process of care should include the identification and elimination of any predisposing factors affecting the pH of the oral environment. The pH of the oral environment is the single most contributory factor to hypersensitive cervical and occlusal surfaces. This is due to both exogenous and endogenous contributing factors.
Any food substance with a critical pH value of less than 5.5 can become a corrosive and demineralize the tooth structure creating erosion. Enamel is comprised of 96% mineralized substrate with 4% being water and organic protein. Under normal conditions with an oral environment of pH greater than 5.5, the enamel is very resistant; however, once compromised by low pH, it becomes vulnerable to abrasion, attrition, and even abfraction. Dentin will demineralize earlier due to its composition.
The role that erosive agents play in the development of dentin hypersensitivity is well established. Erosion of the dentin appears to bring about a rapid loss of the smear layer and the opening of dentinal tubules. Those foods and beverages with a low pH readily remove the smear layer after a few minutes of exposure. Studies suggest that athletes may be particularly at risk for tooth wear and acid erosion due to the more recent emergence and popularity of acidic sports and energy drinks. Attention should be given to obtaining a dietary history in order to identify predisposing acidic factors in the dental hygiene patient’s diet.
Whitening agents may as well induce dentin hypersensitivity. In fact, 55% to 75% of patients undergoing bleaching procedures report sensitivity.11 Proactive desensitization and appropriate selection of an efficacious whitening agent will have a large impact on controlling the sensitivity incurred with professional whitening treatments.
Endogenous or internal contributory factors may include regurgitation associated with gastroesophageal reflux disease or eating disorders, both of which are capable of lowering the pH of the oral environment rapidly and significantly. Xerostomia as a result of the vast array of prescription medications can severely diminish the saliva’s ability to buffer the oral environment.
Oral self-care habits can add another dimension which can be particularly destructive in combination with lowered oral pH. The combination of erosion and abrasion can exacerbate dentin hypersensitivity quickly. Acid studies in vitro suggest that surface softening can extend to 3 to 5 µm and that the tissue is highly susceptible to physical wear.12
SELF-CARE STRATEGIES AND EVIDENCE-BASED RECOMMENDATIONS
Modern lifestyles leave little time for practicing optimum oral health, making dental professionals’ roles even more imperative. Oral self-care can influence the degree of dentin hypersensitivity. Patients who brush excessively or use undue pressure while brushing should be instructed on proper toothbrushing techniques to avoid gingival recession. Tooth brushing (with variations to be considered such as filament stiffness; end rounding; and tooth-brushing force, duration, and frequency) has long since been associated with gingival recession and has been considered relevant.
Also, repeated brushing, immediately following the intake of a low pH food or beverage, can further exacerbate the dissolution of both the enamel and dentin. Wear of enamel and dentin can be dramatically increased if tooth brushing follows an erosive challenge. It is for this reason that the dental patient should avoid toothbrushing for a minimum of 30 to 60 minutes or longer after consuming acidic foods or drinks to reduce the coeffects of acids and abrasion.13
Under normal circumstances, tooth brushing with most toothpaste has little or no effect on enamel and clinically insignificant effect on dentin. Studies in situ, however, suggest that excessive or abusive tooth-brushing habits could cause pathological dentin loss. As a means of comparison, probing force is estimated at 10 to 20 g of pressure with manual toothbrushing ranging from 200 to 400 g and power tooth brushing from 70 to 150 g.3 To place this in perspective, overzealous brushers may utilize 400 to 600 g and even greater when brushing.
An in vitro assessment of dentin wear resulting from the use of oral hygiene devices was conducted by Moore et al.14 The objective was to evaluate dentin wear associated with the use of a power toothbrush and a manual toothbrush using simulated clinical conditions. Brushing loads representing clinical use conditions were established. Samples were brushed either with Philips Sonicare DiamondClean standard and compact brush heads, using externally powered Sonicare handles at 100 g, or with an ADA performed reference manual toothbrush at 250 g brushing loads. Dentin wear was determined before and after brushing using surface profilometry to establish the mean depth of induced surface wear from toothbrushing. Both Sonicare DiamondClean standard and compact brush heads resulted in significantly less dentin abrasion than the manual toothbrush (P < .05). In this in vitro study, The Sonicare DiamondClean was found to cause about 50% less dentin wear than a manual toothbrush.14 These findings become of particular significance when combined with a low pH oral environment.
An in vivo randomized parallel-design study was conducted with an objective to investigate the effects of the Philips Sonicare FlexCare power toothbrush as compared to a manual toothbrush on dentin sensitivity. Sensitivity was assessed on subjects using evaporative and tactile stimulation and defined as experiencing discomfort using a visual analogue scale. The results of the study identified a statistically significant difference between FlexCare and a manual toothbrush following both 4 and 8 weeks of product use after evaporative stimulation. The conclusion of the study was that Philips Sonicare FlexCare showed significant reduction in pain associated with dentin hypersensitivity compared to a manual toothbrush following 8 weeks of product use.15
There have also been subsequent studies designed to compare the long-term effects of using manual and power toothbrushes upon sites of localized gingival recession. No differences at target sites were detected between groups for maximum height of recession, width of keratinized gingiva, clinical attachment level, pocket depth, bleeding on probing (BOP), or plaque index (PI). The study was conducted in vivo involving 52 patients and was a longitudinal, single-blind, randomized, parallel group study. PIs, BOP, and probing depth were recorded at baseline and at 12 months. Maximum height of recession, width of keratinized gingiva, clinical attachment levels, probing depth and PI (Turesky’s Quigley-Hein PI) were recorded for target sites of localized gingival recession at baseline, 3, 6, 9, and 12 months. The findings were noted as follows: no deterioration or progression of localized gingival recession in subjects using the Sonicare Elite compared to a manual over a 12-month period.16
Currently, the paradigm over the past 5 years has been focused on biofilm control instead of total elimination. Even though we discuss the superiority of dental biofilm disruption in power toothbrushing versus manual toothbrushing, it’s important to go one step further and discuss the emerging science of healthy biofilm. The total plaque elimination model is old science. Health is sustainable with incomplete plaque removal. We have come to understand that not all bacteria residing in plaque biofilm is pathogenic. The current paradigm is biofilm control recognizing the ability to deliver beneficial agents into healthy biofilms which can aid in both caries management as well as reducing dentin hypersensitivity. Meticulous, gentle dental biofilm control measures that do not abrade gingival tissues or cervical hard tooth structure are recommended.3
It does appear that the protective and remineralizing nature of the dental biofilm plays a significant role in dentin hypersensitivity by delivery of therapeutic agents designed to diminish hypersensitivity. Diffusion through power toothbrushing was studied by Stoodley et al17 to illustrate the delivery mechanism of a specific dentifrice through the plaque biofilm. The concept was transferred into an in vitro study, where a dual chamber was separated by a membrane colonized with Streptococcus mutans biofilm simulating in vivo interproximal plaque biofilm. The power toothbrushes were placed perpendicular to the biofilm at a 10 mm distance. An 1,100 ppm sodium fluoride was used as is found in the majority of commercial toothpastes. A fluoride electrode in the measurement chamber measured how much fluoride was driven into it through the biofilm membrane following powered brushing in the primary chamber. The diffusion rate coefficient which was a measure of the rate of delivery of fluoride through the biofilm was significant greater (P < .05) than that from passive diffusion alone meaning no brushing. The results of the experiment were published with the Sonicare FlexCare power toothbrush increasing diffusion by 129% over no brushing and by 29% compared to the second power toothbrush utilized. This study demonstrated that the fluid dynamic action of Sonicare FlexCare power toothbrush enhances the penetration of fluoride through biofilm which may, in turn, help increase the bioavailability of fluoride in residual dental plaque.17
The opportunity for delivering and retaining other broad-based antimicrobial or remineralization agents into dental plaque biofilms should be considered in developing novel innovative approaches to dentin hypersensitivity as well as caries management.
CONCLUSION
With the acquired knowledge of the role that causative factors play in localizing and initiating dentin hypersensitivity, prevention strategies may now be included in the dental hygiene care plan. Active management will involve a combination of chairside and oral self-care therapies. Multiple therapeutic modalities are designed to treat dentin hypersensitivity, including products to impede nerve conduction of the pain stimulus, products to mechanically occlude dentin tubules, and calcium-containing products designed to generate plugs in the tubules utilizing a remineralization mechanism.
Although dentin hypersensitivity is not the enigma it once was, there is still much to learn about the condition itself, the management of risk, and prevention behaviors. Our profession is strategically positioned to not only evaluate but also to educate to improve the comfort and quality of life for our dental hygiene patients.
References
- Addy M. Dentine hypersensitivity: new perspectives on an old problem. Int Dent J. 2002;52:367-375.
- Orchardson R, Collins WJ. Clinical features of hypersensitive teeth. Br Dent J. 1987;162:253-256.
- Tillis TS, Keating JS. Dentin hypersensitivity. In: Wilkins EM. Clinical Practice of the Dental Hygienist. 9th ed. Baltimore, MD: Lippincott Williams & Wilkins; 2005.
- Brännström M. Etiology of dentin hypersensitivity. Proc Finn Dent Soc. 1992;88(suppl 1):7-13.
- Brännström M. A hydrodynamic mechanism in the transmission of pain-produced stimuli through the dentin. In: Anderson DJ. Royal Society of Medicine. Sensory Mechanisms in Dentine: Proceedings of a Symposium, London, September 24th, 1962. Oxford, England: Pergamon Press; 1963:73-79.
- Rees JS, Addy M. A cross-sectional study of dentine hypersensitivity. J Clin Periodontol. 2002;29:997-1003.
- Dentin.en.wikipedia.org/wiki/dentin. Accessed September 6, 2011.
- Yoshiyama M, Noiri Y, Ozaki K, et al. Transmission electron microscopic characterization of hypersensitive human radicular dentin. J Dent Res. 1990;69:1293-1297.
- Absi EG, Addy M, Adams D. Dentine hypersensitivity. A study of the patency of dentinal tubules in sensitive and non-sensitive cervical dentine. J Clin Periodontol. 1987;14:280-284.
- Addy M, Mostafa P, Newcombe RG. Dentine hypersensitivity: the distribution of recession, sensitivity and plaque. J Dent. 1987;15:242-248.
- Haywood VB. Treating sensitivity during tooth whitening. Compend Contin Educ Dent. 2005;26(9 suppl 3):11-20.
- Eisenburger M, Hughes J, West NX, et al. Ultrasonication as a method to study enamel demineralisation during acid erosion. Caries Res. 2000;34:289-294.
- Canadian Advisory Board on Dentin Hypersensitivity. Consensus-based recommendations for the diagnosis and management of dentin hypersensitivity. J Can Dent Assoc. 2003;69:221-226.
- Moore M, Putt M, Jain V, et al. In vitro assessment of dentin wear resulting from the use of the Philips Sonicare DiamondClean power toothbrush. Koninklijke Philips Electronics N.V. Data on file, 2010.
- Frederick C, DeLaurenti M, Olson M, et al. Comparison of Sonicare FlexCare power toothbrush and a manual toothbrush on dentin hypersensitivity. Koninklijke Philips Electronics N.V. Data on file, 2009.
- McCracken GI, Heasman L, Stacey F, et al. Changes in localized gingival recession with manual and powered toothbrushes. Paper presented at: IADR/AADR/CADR 87th General Session and Exhibition; April 4, 2009; Miami, FL. Abstract 3505.
- Stoodley P, Nguyen D, Longwell M, et al. Effect of the Sonicare FlexCare power toothbrush on fluoride delivery through Streptococcus mutans biofilms. Compend Contin Educ Dent. 2007;28(suppl 1):15-22.
Ms. Jones is an international speaker for the profession of dental hygiene and president of RDH CONNECTION Inc, a consulting and training company dedicated to excellence in quality dental hygiene education. Having a career that has spanned more than 3 decades, her experience has encompassed clinical practice, education, and international lecturing, and she has published internationally. She has been appointed to serve on Dentistry Today‘s advisory board and joins its 2012 Leaders in Continuing Education. She can be reached at jjones@rdhconnection.com or at the Web site rdhconnection.com.
Disclosure: Ms. Jones has received an educational grant from Philips Sonicare towards the writing of the article.