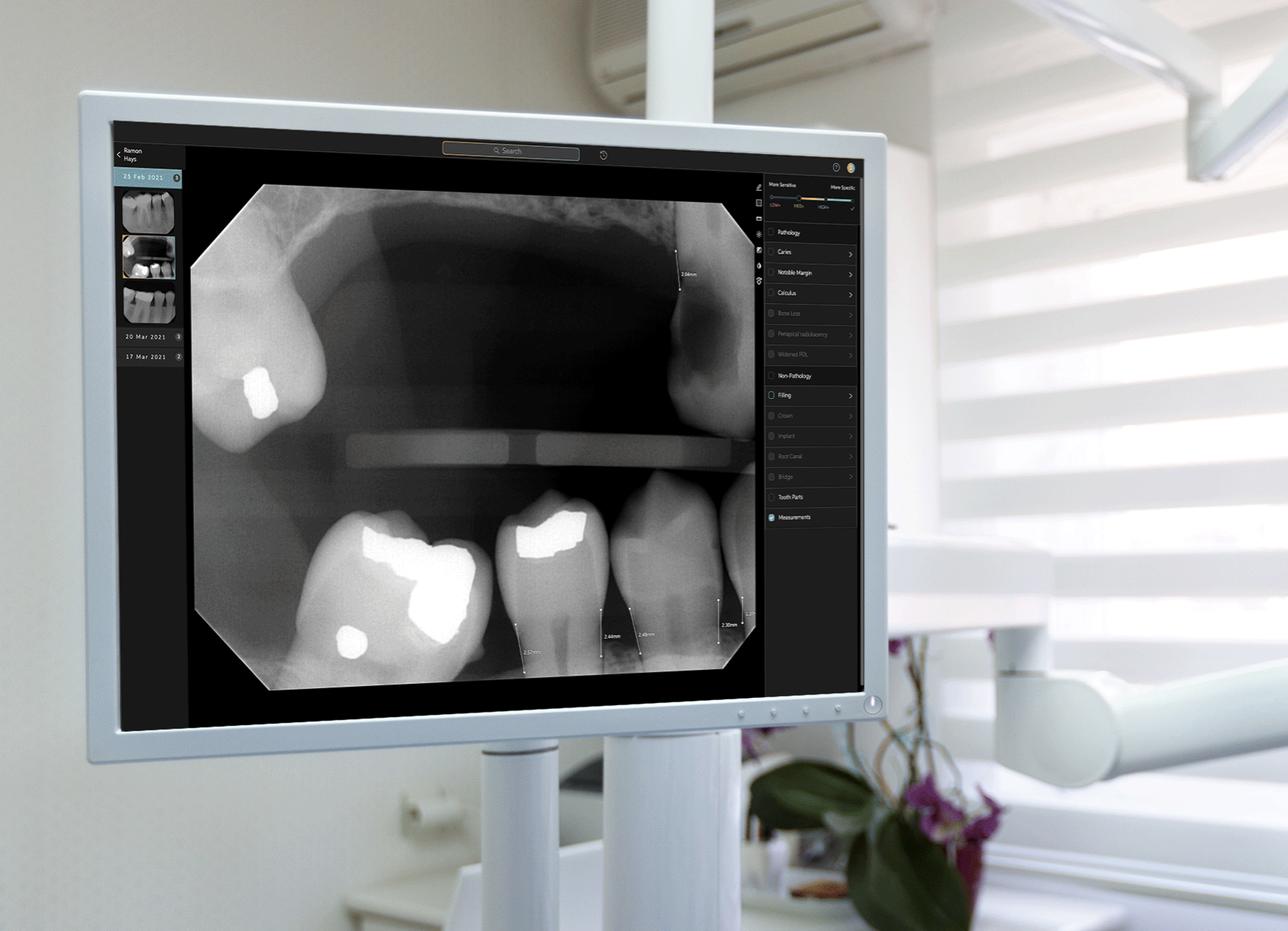
Pearl, the leader in dental AI solutions, has announced that the United States Food and Drug Administration (FDA) cleared its AI-powered, real-time pathology detection solution Second Opinion to help dentists accurately identify a broad range of common dental conditions in patient x-rays.
Pearl’s announcement marks an important step forward for technology-assisted dental care. Second Opinion becomes the first AI-driven solution cleared to detect numerous conditions in dental x-rays, and the first to add US FDA clearance to European CE, Canadian MDEL, UAE Ministry of Health and Prevention, Australian TGA and New Zealand MEDSAFE clearances.
Second Opinion is now the only AI-driven computer-aided clinical radiology solution available to dentists throughout North America, Europe, Australia and many other parts of the world.
“This clearance is a major milestone not only for our team and for the many dentists, advisors and partners who have contributed to Second Opinion’s development, but also for dentistry itself,” said Ophir Tanz, CEO and founder of Pearl. “AI is a paradigm-shifting technology that will add value across the entire healthcare continuum. Because x-rays are a regular part of every dental patient’s experience, the first place most people will encounter the power of medical AI technology will be in their dentist’s chair. Second Opinion’s FDA clearance has made that possible.”
Pearl’s system supports dental professionals in their review of radiographs by applying computer vision to identify and highlight key pathologic and nonpathologic findings, including dental caries, discrepancies at the margin of existing restorations, calculus, periapical radiolucency, crowns, fillings, root canals, bridges and implants.
When x-rays are captured in a dental office, Second Opinion® immediately displays the images and any detected conditions on monitors in the operatory, giving dentists sharper vision into their patients’ oral health and giving patients greater understanding of their dentist’s findings.
“The benefit that Pearl’s AI brings to patient communication in the dental operatory – and the trust that follows – cannot be overstated, and it is in that area that Second Opinion’s impact will be most immediately felt,” said Professor Dr. Markus Blatz, DMD, a leading authority in restorative dentistry and digital innovation. “Its long-term impact on the field of clinical dentistry may be more important, however. We endeavor to establish a standard of care through instruction at dental school, but at some point our instruction stops. Second Opinion will help us maintain that standard and, over time, establish a more consistently rewarding patient experience.”
Second Opinion exceeded the FDA’s stringent efficacy requirements across four separate clinical studies, each featuring a radiographic dataset of over 2,000 images and a reader group of 86 expert dentists and dental radiologists.
The tests showed clear advantages for readers using Second Opinion, including finding that study participants reading x-rays with the assistance of Pearl’s AI system accurately identified 36% more lesions than those without AI assistance did.
“Second Opinion now joins a family of FDA cleared CADe medical systems already in use for radiologically-driven tasks such as lung nodule detection and mammography interpretation,” Pearl’s Chief Technology Officer and co-founder, Cambron Carter, said. “State of the art algorithms that currently assist in the detection of cancerous lesions can now be applied to detect many more frequently occurring dental diseases. The standard of care in dentistry is about to level up.”
Pearl’s AI driven software is already in daily use by radiograph manufacturers, dentists and dental service organizations across Europe, Canada, Australiasia, South America and the Middle East.
US dentists and dental offices can begin implementing Second Opinion today by visiting: www.hellopearl.com/products/second-opinion.
About Pearl
Pearl is shaping the future of dental care by delivering AI and computer vision solutions that advance efficiency, accuracy, transparency and patient care. Founded in 2019 by Ophir Tanz, Pearl is backed by Craft Ventures and other leading venture capital firms.
For more information or to request a demonstration, please visit https://www.hellopearl.com.