Scrubbing has long been the central strategy for cleaning teeth. The bristles of toothbrushes and the silica in toothpastes treat the teeth much the same as we would treat a shower or sink that needs cleaning, even as the mouth is a complicated system made up of a variety of oral tissues. The untargeted approach of abrasion-based oral care can often result in damage to tooth structure, impeding the remineralization of the enamel and diminishing one’s overall oral health. A modern understanding of plaque and the physics of how it attaches to teeth leads us to more gentle and effective methods of preventing plaque accumulation. Developments in the application of chelation—the binding of ions and molecules to metal ions—provide an unprecedented level of targeted, effective, and gentle oral care. Chelating agents in toothpastes, used at appropriate levels, can remove and prevent the buildup of undesirable bacterial plaque while leaving healthy levels of desirable calcium, achieving the chemical balance that is foundational to oral health.1
Bacterial plaque, as well as the formation of calculus on teeth and around the gingiva, has been intricately linked to the development of oral diseases such as caries, gingivitis, and periodontitis.2 Hence, plaque and calculus control are considered crucial to the effective self-management and prevention of oral disease development. Clinicians often counsel their patients to carefully remove plaque via the physical action of tooth brushing; however, the chemistry of plaque, calculus, enamel, dentin, and saliva constitute an intricate relationship. Only a handful of elements are present at the tooth surface that allow plaque to accumulate. Calcium is one of those elements. It plays a vital role in the development of both dental biofilm and calculus3 (ie, when calcium is in the wrong place at the wrong time) while also serving as the main atomic building block of enamel and dentin (ie, when calcium is in the right place at the right time).
Both hydroxyapatite (HA) and the substances that form upon it contain the same 2 molecules: calcium phosphate and hydroxyl ions. Even if the teeth are completely free of plaque, calcium ions in the dental enamel are continually removed and added in a dynamic demineralization-remineralization process known as the “demin-remin cycle.” This cycle occurs because food and drinks contain corrosive, calcium-depleting acids that give the food and drinks flavor. Fortunately, humans have saliva to protect enamel and replenish the healthy calcium and phosphorus diminished by that corrosion. Salivary proteins ensure that saliva is supersaturated with calcium phosphate; armed with an excess of these mineral ions, healthy saliva drives remineralization by replenishing calcium and phosphate lost from the tooth surface. Healthy saliva also creates what is called the “acquired enamel pellicle,” which serves to further protect the enamel and acts as an ion diffusion barrier.
While some intraoral bacteria can form a mutually symbiotic, healthy biofilm that confers resistance to disease, oftentimes, the biofilm that is closest to the tooth surface becomes detrimentally acidic, thereby creating an environment that dissolves tooth structure. Without chemical neutralization or the removal of that acidic biofilm, the tooth surface becomes dysbiotic and may begin to demineralize, creating an initial caries lesion.
Unremoved non-acidic plaque has an opposite, though still detrimental, effect. When it is bathed in calcium- and phosphate-rich saliva, the non-acidic plaque calcifies due to the ions deposited within it. Thus, both acidic and non-acidic plaque damage gums and play key roles in the development of receding gums, gingivitis, and periodontitis.4
Anti-calculus agents are used extensively in toothpaste to delay and interfere with dental plaque calcification. The most common anti-calculus agents are metal chelators.1 Chelation is a chemical reaction in which ions and molecules (ligands) become bonded to metal ions; this bond involves the formation or presence of 2 or more separate coordinate bonds between a polydentate (multiple-bonded) ligand and a single central metal atom. Essentially, the chelator is a molecule designed to grab onto a metal ion.
The most common anti-calculus chelators include ethylenediaminetetraacetic acid (EDTA), sodium hexametaphosphates (SHMPs), and pyrophosphates. They all work with essentially the same mechanism: Each has a high affinity to HA surfaces thanks to an interaction with calcium ions (Ca2+) in the hydration layer. In this interaction with HA and enamel surfaces, chelators reduce the protein-binding capacities of these surfaces. They also have the ability to inhibit calcium phosphate formation.5 Essentially, chelators get in the way of the calculus-hardening process: Like adding bowling balls to a brick wall, the calculus structure becomes wobbly and weak and thereby slower to accumulate and easier to remove.
Some chelating processes occur naturally in nature, such as in serum or biological tissues. Chelators have also been developed as tools for a wide range of industrial applications, including water softening and food preservation. Their ability to bind to and remove metal ions, especially unwanted calcium, means that chelators also have many important medical applications, as chelating effects can be achieved at a near-neutral pH.6 Chelators are already commonly used in dentistry; endodontic instrumentation relies upon chelators to facilitate canal cleaning by binding to calcium to assist in its removal. In addition, chelators are often the active ingredient in tartar-control toothpastes, where they bind to excess salivary calcium ions in order to prevent those ions from precipitating and forming dental calculus.1 It is important to note, too, that the efficacy of chelators in tartar-control toothpaste is predicated upon the fact that one usually brushes twice a day for roughly 2 minutes, which means that chelators from toothpaste are only active in the mouth for about 4 minutes in an entire day. This relatively brief timespan ensures the safety of chelating agents in that they are not removing too much calcium from the mouth, allowing the vast majority of calcium ions necessary for oral health to remain (Figures 1 and 2).


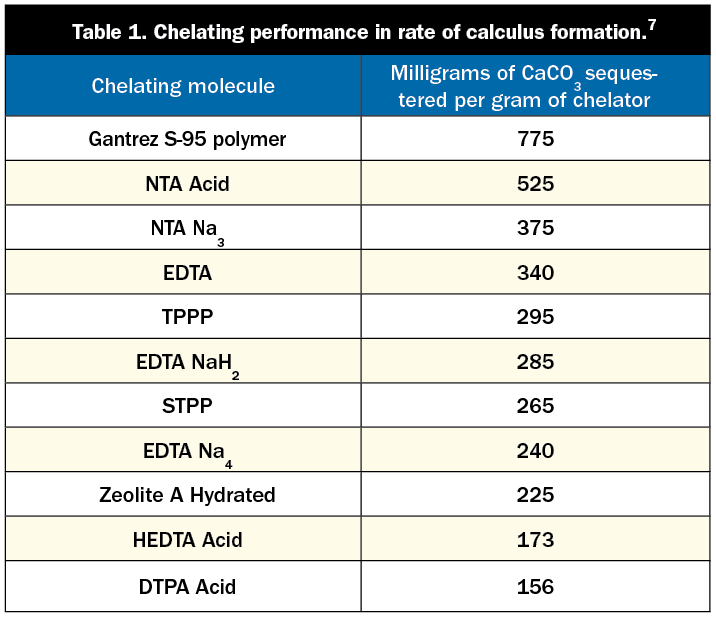
There are differences in the amount of chelation achieved by the various leading chelators used in tartar-control toothpastes. The most commonly used chelators for calculus control are pyrophosphates, SHMPs, Gantrez (a copolymer of maleic acid), and EDTA. Each of these compounds is safe and effective in binding to calcium and other metals. Each chelator has its own affinity for metal ions (this affinity is a measure of its ability to hold on to a metal ion once it is bound). Table 1 shows the varying levels to which these chelators reduce the rate of calculus formation.
Studies have shown that pyrophosphates reduce the rate of dental calculus formation by around 30%, while SHMP reduces it by up to 50%.8
Colgate Total with Triclosan contained 2% Gantrez, a maleic acid copolymer.3 Gantrez is used in dentifrices and is described as a “bioadhesive polymer”4 that adheres to oral surfaces. According to Ashland Corp, it “function(s) by chelating metal ions….Calcium phosphate occurring naturally in the mouth normally forms a pre-calculus or tartar seed on the teeth….[Gantrez] attacks the tartar seed and the seed dissolves.”9 The dissolution of the pre-calculus is achieved by chelating the calcium.
EDTA disodium is another well-known chelator that has been extensively studied since its development by Ferdinand Munz in Germany in the early 1930s. EDTA is commonly used as a chelator added to many food items as a preservative. In high concentrations (18% to 25%), it is useful in removing smear layers during root canal preparation.
Given the safety and efficacy of chelators, research has explored ways to implement chelation in methods of plaque control that are even more targeted and effective than conventional uses of chelation. One major development has involved using chelators to strengthen the natural negative electronic charge of the tooth surface to repel unhealthy calcium, thereby achieving the dual aims of making unwanted calcium in the plaque fluid (calcium in the wrong place at the wrong time) easy to remove from the tooth environment without getting rid of the calcium necessary for oral health.
Tooth surfaces are negatively charged, and so are bacteria; therefore, they should repel each other. However, salivary calcium coats the negative charges on the tooth surface and bacteria, allowing them to get very close (within 10 nm). At this point, van der Waal’s forces (attractive electrostatic forces at small distances) take over, allowing the bacteria to deposit on the tooth surfaces, initiating biofilm formation.10 A unique formulation of EDTA strengthens the negative electronic forces of the tooth, allowing the teeth to repel harmful plaque. This special formulation quickly penetrates through the plaque down to the tooth surface. There, it changes the surface charge back to negative by neutralizing the positively charged calcium ions. This new, stronger negative charge on the tooth surface environment simply allows the plaque and the tooth surface to repel each other. This requires neither an abrasive nor killing the bacteria (Figure 3).
This specialized formulation of 2.6% EDTA, which is currently available in LIVFRESH Dental Gel (Livionex), not only repels the bacteria but also remains on the tooth surface—a characteristic known as substantivity—and slows down the rate of future bacterial attachment to that tooth surface, reducing the rate of plaque buildup. In addition, the increased negative charge of the tooth surface weakens the attachment of plaque to the tooth surface, making it easier to remove the next time the tooth is brushed. Brushing at night with this formulation of 2.6% EDTA has been shown to significantly lower plaque buildup overnight.11 This “smart technology” mechanism effectively controls plaque and tartar buildup without the need for abrasives, soaps, and bactericidal chemicals (Figures 4 and 5).
As Table 2 shows, the per-brush chelating capacity of LIVFRESH Dental Gel is extraordinarily targeted and is less than 10% of the chelating capacity of the 13% SHMP used in Crest Pro-Health.
The amount of toothpaste used in a single brushing of Colgate Total or Crest Pro-Health would chelate 6 or 13 times the amount of calcium, respectively, that would be chelated by the same amount of LIVFRESH Dental Gel used in a single brushing, even as LIVFRESH has shown remarkable efficacy in the removal of plaque. In multiple controlled studies, the use of the uniquely formulated 2.6% EDTA has consistently resulted in lower plaque, gum inflammation, and bleeding compared to other toothpastes.15-17 Most recently, a double-blind study published in the Journal of Periodontology confirmed that, without prophylaxis, 2.6% EDTA showed statistically and clinically significant reductions in pocket depths and gum inflammation and bleeding compared to a leading stannous fluoride-containing anti-gingivitis toothpaste in early/moderate (stage 1 and stage 2) periodontitis patients.18
CONCLUSION
The selectively focused use of chelators has the potential to effectively control dysbiotic oral diseases by increasing the negative charge of teeth to repel plaque naturally. At the same time, this novel approach is respectful and supportive of healthy oral flora. Repurposing a safe, popular tartar-control agent to repel plaque offers an exciting future for more effective home care that does not rely on abrasives and antimicrobials.
REFERENCES
1. Koenig P, Faller R. Fundamentals of dentifrice: oral health benefits in a tube. Updated June 28, 2020. Available at: https://www.dentalcare.com/en-us/professional-education/ce-courses/ce410
2. American Dental Association (ADA) Division of Science. For the patient. Keeping your gums healthy. J Am Dent Assoc. 2015;146(4):A46. doi:10.1016/j.adaj.2015.01.021
3. Jin Y, Yip HK. Supragingival calculus: formation and control. Crit Rev Oral Biol Med. 2002;13(5):426-41. doi:10.1177/154411130201300506
4. Damle SG. Genetic determination through dental calculus: Promise and hope! Contemp Clin Dent. 2016;7(2):129-30. doi:10.4103/0976-237X.183065
5. Vranić E, Lacević A, Mehmedagić A, et al. Formulation ingredients for toothpastes and mouthwashes. Bosn J Basic Med Sci. 2004;4(4):51-8. doi:10.17305/bjbms.2004.3362
6. Levine RS. Pyrophosphates in toothpaste: a retrospective and reappraisal. Br Dent J. 2020;229(10):687-689. doi:10.1038/s41415-020-2346-4
7. Davidsohn AS, Milwidsky B. Synthetic detergents. 7th ed. Longman Scientific & Technical; 1987.
8. Schiff T, Saletta L, Baker RA, et al. Anticalculus efficacy and safety of a stabilized stannous fluoride/sodium hexametaphosphate dentifrice. Compend Contin Educ Dent. 2005;26(9 Suppl 1):29-34.
9. Ashland Corporation. Gantrez S polymers for tartar control in toothpastes and mouth rinses. Bulletin VC-814.
10. Hermansson M. The DLVO theory in microbial adhesion. Colloids and Surfaces B: Biointerfaces. 1999;14(1–4):105-119.
11. Anbarani AG, Wink C, Ho J, et al. Dental plaque removal and re-accumulation: a clinical randomized pilot study evaluating a gel dentifrice containing 2.6% edathamil. J Clin Dent. 2018;29(2):40-44.
12. Kozak, KM, White DJ. Poster 2086: Dentifrice effects toward chemical stain prevention: An in vitro comparison. 2000. Poster 2086; Presented at 78th General Session and Exhibition of International Association for Dental Research (IADR).
13. Schiff T, Saletta L, Baker RA, et al. Anticalculus efficacy and safety of a stabilized stannous fluoride/sodium hexametaphosphate dentifrice. Compend Contin Educ Dent. 2005;26(9 Suppl 1):29-34.
14. Ashland Corporation. Gantrez TM-95 polymer building performance for non-phosphate dishwashing products. Ashland Product Sheet, PC 7822.
15. Dadkhah M, Chung NE, Ajdaharian J, et al. Effects of a novel dental gel on plaque and gingivitis: a comparative study. Dentistry (Sunnyvale). 2014;4(6):239. doi:10.4172/2161-1122.1000239
16. Ajdaharian J, Dadkhah M, Sabokpey S, et al. Multimodality imaging of the effects of a novel dentifrice on oral biofilm. Lasers Surg Med. 2014;46(7):546-52. doi:10.1002/lsm.22265
17. Nayudu A, Lam T, Ho J, et al. Plaque removal and gingival health after use of a novel dental gel: a clinical study. Dentistry (Sunnyvale). 2016;6(10):396. doi:10.4172/2161-1122.1000396
18. Kaur M, Geurs NC, Cobb CM, et al. Evaluating efficacy of a novel dentifrice in reducing probing depths in stage I and II periodontitis maintenance patients: a randomized, double-blind, positive controlled clinical trial. J Periodontol. 2021;92(9):1286-1294. doi:10.1002/JPER.20-0721
ABOUT THE AUTHORS
Dr. Jacobsen has a PhD in Comparative Pharmacology and Toxicology, and he directed the Oral Medicine Clinic at the University of the Pacific, Arthur A. Dugoni School of Dentistry in San Francisco for 25 years. He is a Diplomate of the American Board of Oral Medicine and a past chairman and vice-chairman of the ADA Council on Scientific Affairs. He has been named as one of Dentistry Today’s Leaders in Continuing Education for several years. He also received the 1999 Gordon J. Christensen Lecturer Recognition Award. He writes the Dental Drug Booklet, a succinct handout and reference on commonly prescribed dental medications. Dr. Jacobsen lectures extensively on dental pharmacology as well as over-the-counter dental drugs and products. He also presents on the topic of the dental management of medically complex patients. He can be reached at pgjacobs@pacbell.net.
Dr. Nový is the chief dental officer of the Alliance Dental Center, Massachusetts Public Employees Fund, and holds faculty appointments at the Harvard School of Dental Medicine and Western University. He served on the ADA Council of Scientific Affairs from 2011 to 2014 and as president of the National CAMBRA Coalition. In 2016, he was appointed the consumer representative to the US Food and Drug Administration Dental Products Panel. He is the recipient of the Dugoni Award, the Weclew Award, and the 2021 ADA Evidence-Based Dentistry Practice Award. He can be reached at drbriannovy@gmail.com.
Disclosure: Drs. Jacobsen and Nový are members of the Livionex Scientific Advisory Board.