The National Institute of Dental and Craniofacial Research (NIDCR) has awarded GreenMark Biomedical a $1.5 million Phase II Small Business Innovation Research (SBIR) grant to further develop its patented nanoparticle-based dental technology for detecting early stage dental caries that could be treated noninvasively without fillings or surgery.
The two-year project involves collaboration with dental and biomaterials experts at the University of Michigan, Creighton University, Tufts University, and the University of Colorado. GreenMark’s technology uses bioresorbable starch-based nanoparticles that degrade into harmless materials by the time the patient leaves the dental office.
“Effective management of dental caries, which affects well over 90% of the world’s population, is characterized by detection of early lesions and accurate diagnosis of caries activity,” said Brian Clarkson, BChD, LDS, MS, PhD, professor with the Department of Cariology, Restorative Sciences and Endodontics, at the University of Michigan School of Dentistry.
“Patients are currently failing to benefit from the scientific developments supporting noninvasive dentistry. GreenMark and their collaborators are helping to enable this advance in dentistry,” said Clarkson.
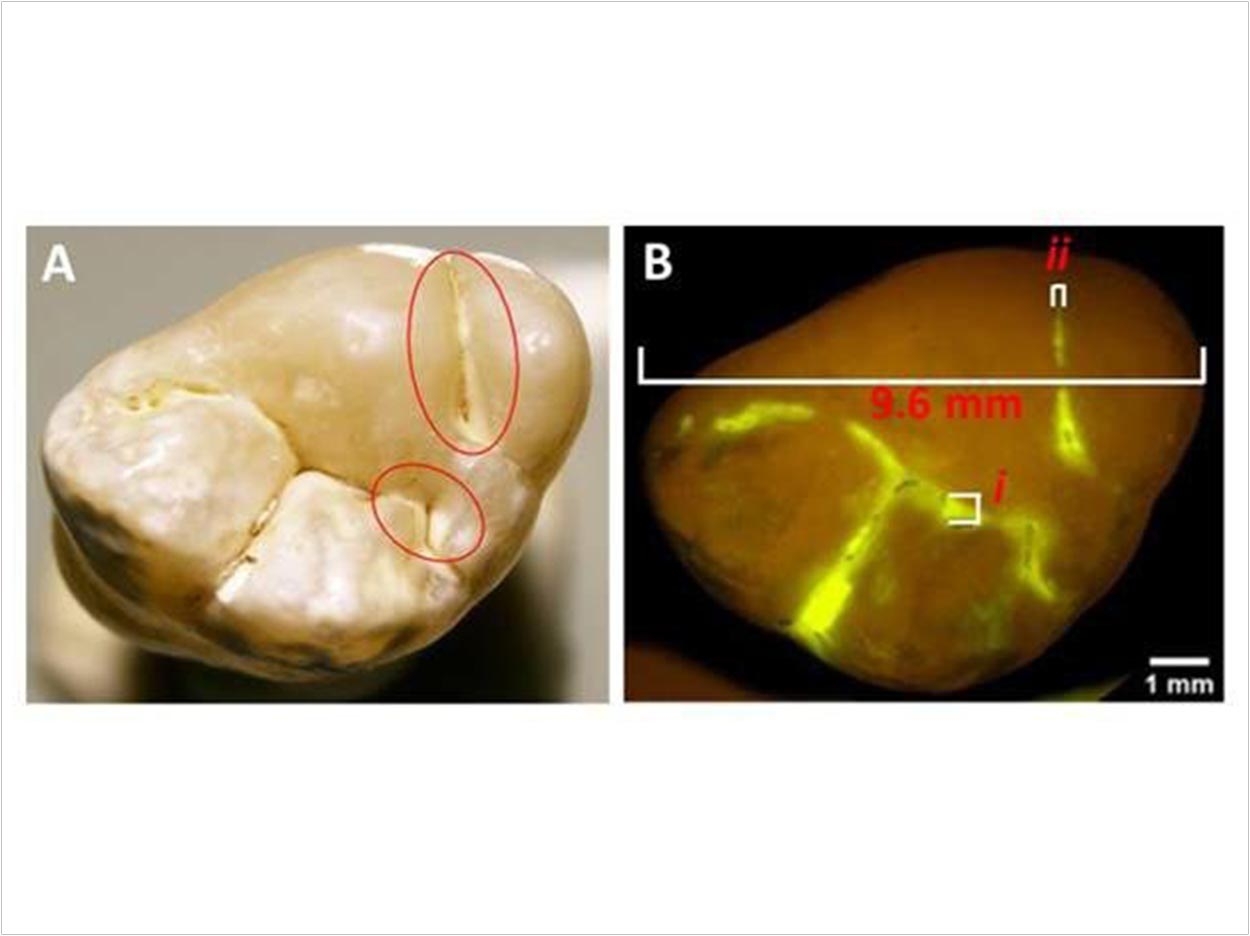
According to GreenMark, small particles produced from food-grade starch make an ideal carrier for dental applications, since enzymes in the body and saliva degrade starch. The company’s technology includes fluorescently labeled starch particles that target active caries and illuminate them using a standard curing light.
By identifying caries before cavitation, clinicians can pursue nonsurgical management options, resulting in less discomfort and improved long-term oral health outcomes for patients, GreenMark says.
The company’s researchers also have demonstrated the ability to load the essential minerals, depleted as a result of tooth decay, directly inside the small starch particles. Unlike fluoride products, which seal the tooth’s enamel surface, GreenMark’s treatment products are designed to target the enamel surface.
“We are excited by our SBIR grant award and the assembly of this team of experts, which will benefit our preclinical testing and clinical validation tremendously,” said Wendy Bloembergen, MD, MS, GreenMark’s vice president of clinical affairs.
Related Articles
Cavity Identification Technology Finds More Equity Funding Support
Device Detects Cavities Before an X-Ray Can
Demineralization Detector Begins FDA Approval Process


