INTRODUCTION
Guided bone regeneration (GBR) is the most common method used for bone augmentation in implant dentistry. GBR utilizes a barrier membrane to occlude soft-tissue cells and allow slower growing bone cells to repopulate the defect and regenerate bone.1 Additional principles for successful GBR include tension-free primary closure of the flap over the site, angiogenesis during healing to provide the necessary blood supply and undifferentiated mesenchymal cells, space creation, and maintenance for bone ingrowth, and stability of the wound during healing.2 The type of membrane used for GBR is based on several factors, including the morphology of the osseous defect, rigidity for space maintenance, barrier function, and handling characteristics. Another important consideration is the type of bone graft material used under the membrane. This article will discuss characteristics of GBR membranes that direct their use for the treatment of various bone defects.
Membranes for GBR
Proper membrane selection is critical for optimal bone regeneration. There are several membranes available today, each with distinctive characteristics that should be considered to optimize clinical performance and improve outcomes. Membranes can generally be classified as either non-absorbable or absorbable. Non-absorbable membranes remain intact during healing and bone regeneration. They exhibit biocompatibility, exceptional mechanical strength, increased rigidity, and favorable space maintenance. These features make them ideal for horizontal and vertical bone augmentation, especially when managing non-space-making defects. However, this type of membrane requires a second surgical procedure for removal and is more technically challenging to use as it requires rigid fixation with tacks or screws. They also have a higher risk of exposure to the oral environment, thus increasing the risk of secondary infection and diminished bone regeneration.3
 |
 |
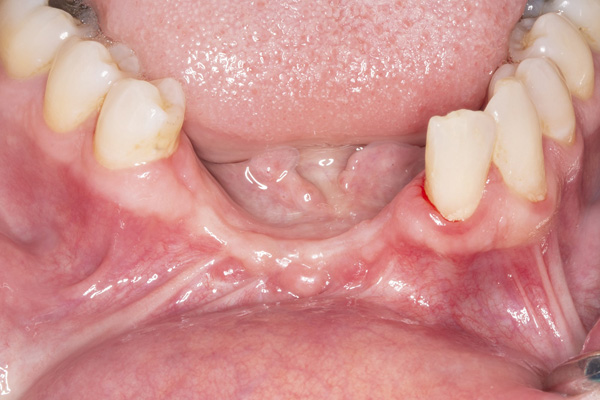
| Figure 1. Preoperative view of 2 failing dental implants replacing the mandibular incisors. | Figure 2. A periapical radiograph reveals significant bone loss around the implants and adjacent teeth. |
 |
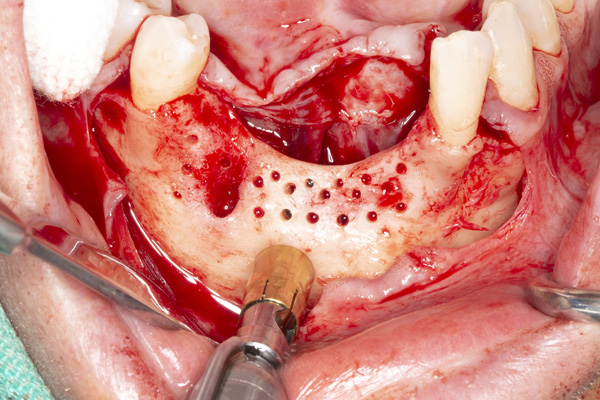 |
| Figure 3. The healed ridge after implant removal. | Figure 4. The ridge is exposed for cortical perforations and harvesting autogenous bone. |
Absorbable membranes were developed to overcome some of the disadvantages of non-absorbable membranes. Collagen-based products are the most commonly used absorbable membranes. Absorbable collagen membranes have several advantages, including ease of handling, improved soft-tissue healing, and lower risk of membrane exposure.4 They also decrease patient morbidity as they do not require retrieval after healing. The disadvantages of collagen membranes include a lack of space maintenance and a more limited barrier function time compared to non-absorbable membranes.
 |
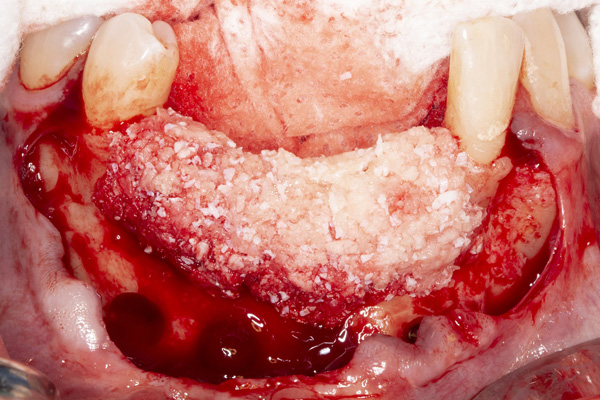 |
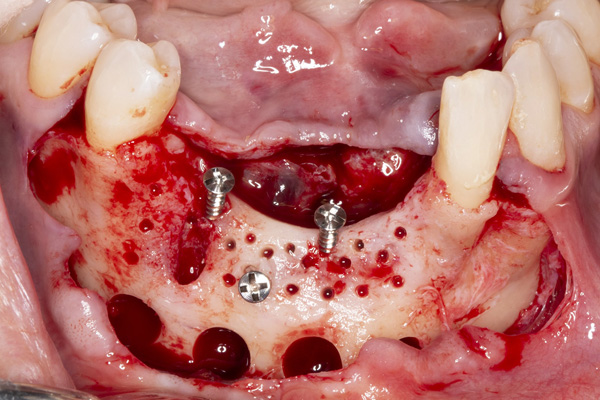
| Figure 5. Tenting screws were placed in the anterior mandible to support the graft. | Figure 6. The ridge was augmented with particulate autograft, bovine bone mineral, and platelet-rich fibrin. |
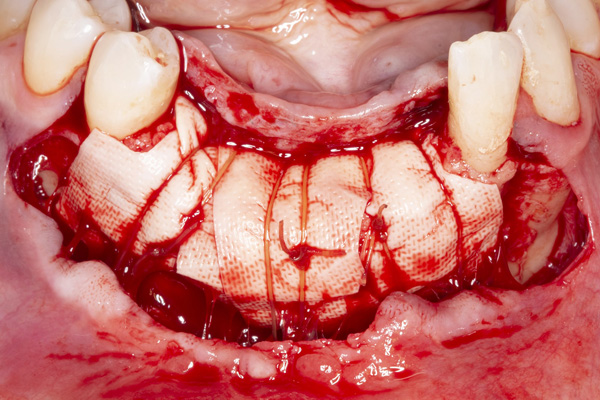 |
 |
| Figure 7. The collagen membrane is secured with periosteal sutures. | Figure 8. The reconstructed ridge after 6 months of healing. |
Absorbable collagen membranes can be made from native collagen or artificially cross-linked. Commercially available collagen membranes provide different resorption times based on the type of collagen and the cross-linking process. It is important to select a membrane that maintains its structural integrity for the time necessary to allow the proliferation and maturation of bone cells within the defect. Although native collagen membranes have desirable handling properties and incorporate well into the tissue bed, this type of membrane degrades more rapidly.5 Cross-linking the collagen fibers slows the rate of degradation and maintains structural integrity to prolong barrier function. This feature is important when greater osseous gains are required as longer barrier function is necessary to allow proliferation and maturation of the bone-forming cells. Cross-linking can be produced by either chemical or physical reagents. One of the most widely performed techniques is the use of glutaraldehyde.4 However, glutaraldehyde cross-linking has been shown to elicit a greater inflammatory response and is associated with a higher incidence of wound dehiscence and membrane exposure.6-8
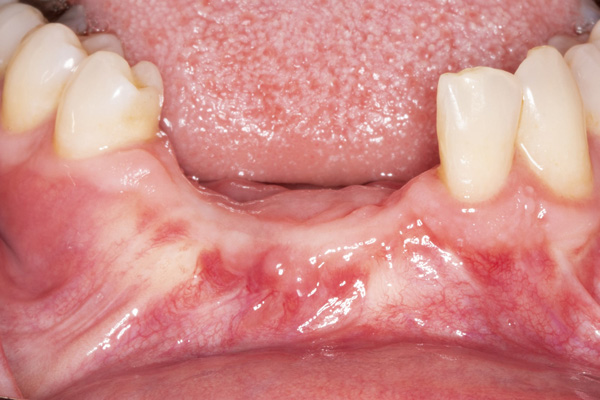
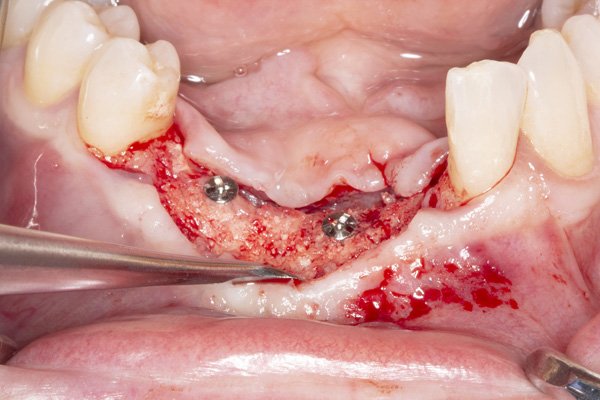 |
 |
| Figure 9. Exposure of the healed ridge reveals bone gain to the level of tenting screws. | Figure 10. An occlusal view of the healed ridge reveals favorable 3D contour. |
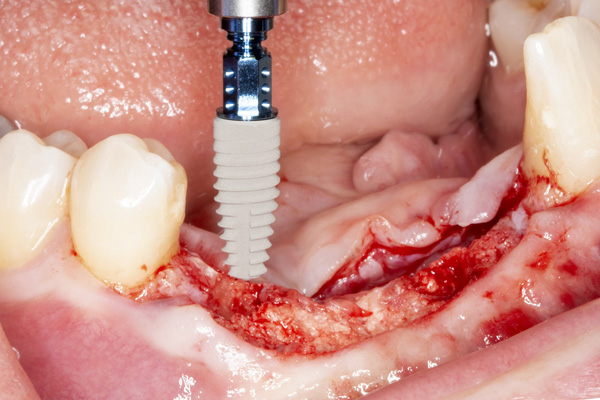 |
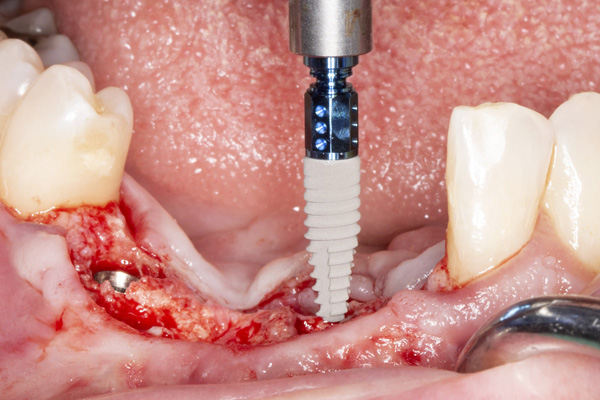 |
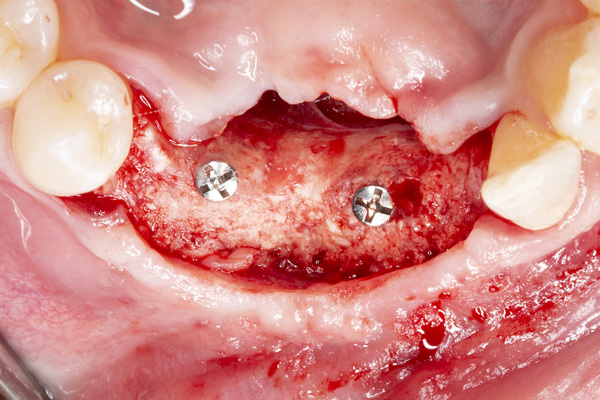
| Figure 11. Placement of implant No. 27. | Figure 12. Placement of implant No. 25. |
Ribose Cross-Link Technology
Glycation uses a non-toxic, naturally occurring sugar (ribose) to cross-link the collagen fibrils while maintaining a high level of biocompatibility. OSSIX Plus collagen membrane (Dentsply Sirona) is an example of an absorbable ribose cross-linked collagen membrane that uses glycation through Dentsply Sirona’s patented GLYMATRIX technology. This membrane is one of the most extensively researched, with more than 100 scientific publications on its use, and offers several advantages for guided bone regeneration. OSSIX Plus is cross-linked to a much higher degree than native or other cross-linked collagen membranes, resulting in a unique resistance to collagenases and a significantly longer barrier function of 4 to 6 months.9 This characteristic can have significant clinical advantages, particularly in larger, extrabony defects where prolonged barrier function is necessary. In addition, a graft material with greater biologic activity, such as autogenous bone, is often needed for vertical augmentation.10
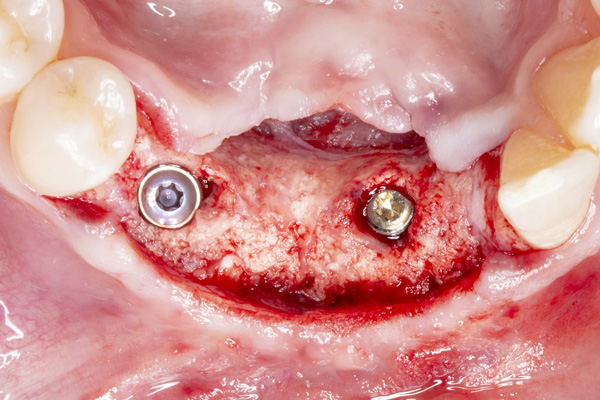 |
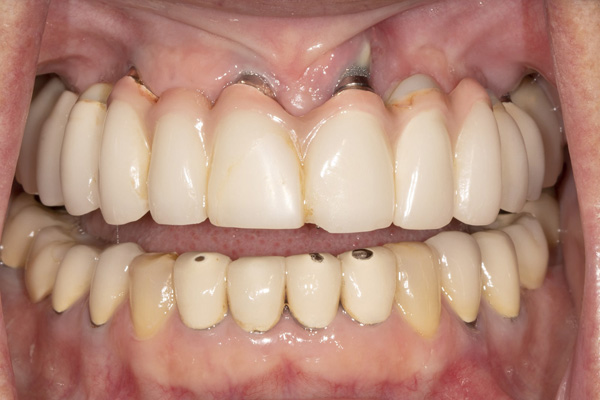 |
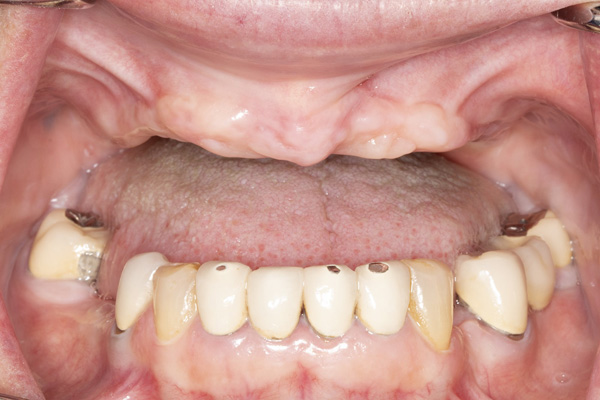
| Figure 13. An occlusal view of the 2 implants in the reconstructed mandible. | Figure 14. Pre-op view of the failed maxillary implants supporting a fixed bridge. |
 |
 |
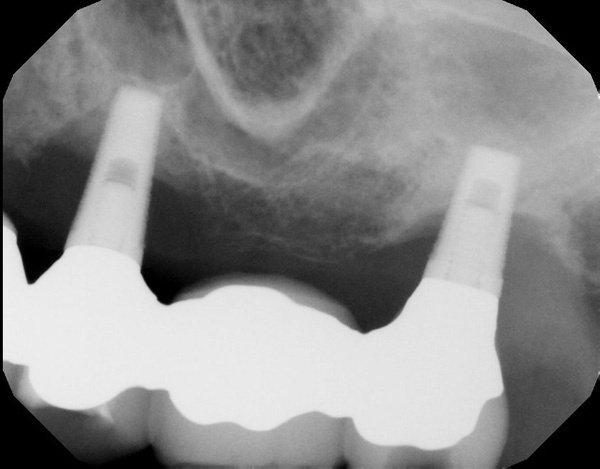
| Figure 15. Periapical radiograph of the maxillary left implant reveals severe marginal bone loss. | Figure 16. The maxillary ridge following healing after implant removal. |
A unique property of the OSSIX Plus membrane is the in situ ossification of the collagen. A human histological case series was the first to demonstrate direct mineral apposition on glycated collagen.11 This has also been confirmed in a case series study that showed collagen and bone apposition on the membrane remnants, further demonstrating this membrane’s high grade of biocompatibility.12 The combination of high biocompatibility and prolonged degradation of the ribose cross-linked membrane results in the integration of the membrane into the local bone as the material serves as a substrate for ossification.
 |
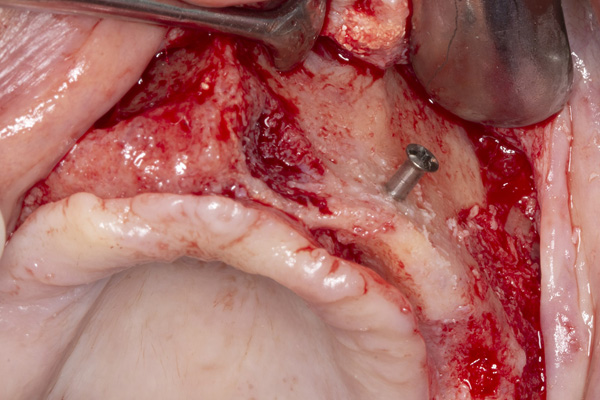 |
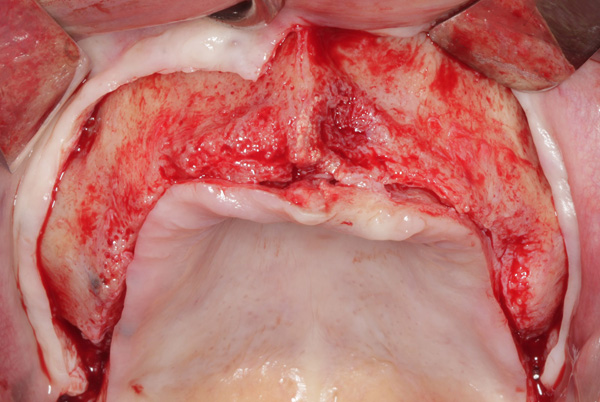
| Figure 17. Exposure of the atrophic maxilla. | Figure 18. A tenting screw is used to support the particulate bone graft and membrane. |
 |
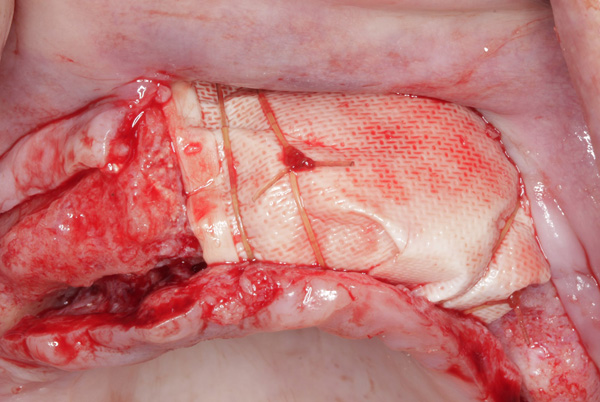 |
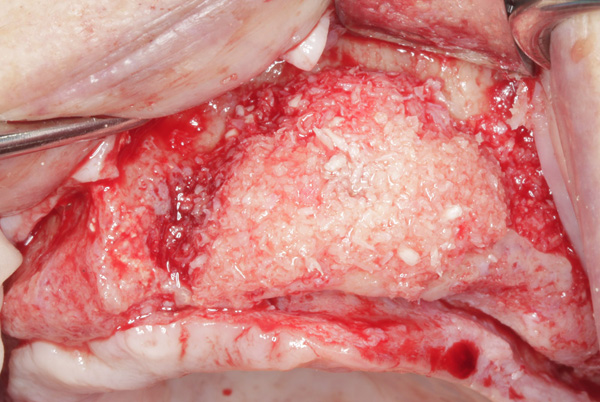
| Figure 19. The ridge was augmented with particulate autograft, bovine bone mineral, and platelet-rich fibrin. | Figure 20. The graft in the left maxilla was covered with collagen membrane and secured with periosteal sutures. |
 |
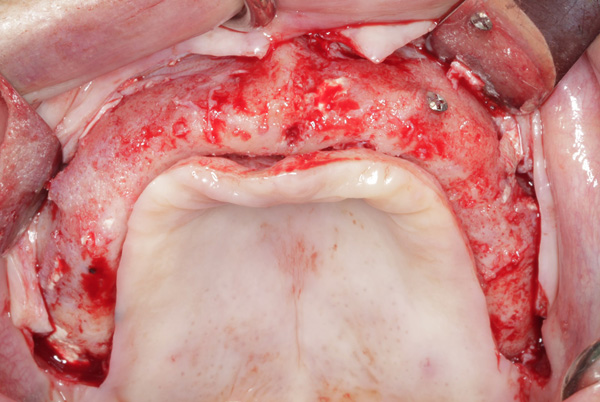 |
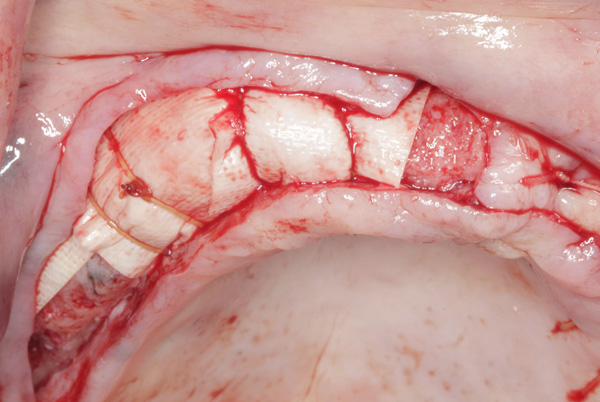
| Figure 21. The graft in the right maxilla was covered with collagen membrane and secured with periosteal sutures. | Figure 22. The reconstructed ridge after 6 months of healing. |
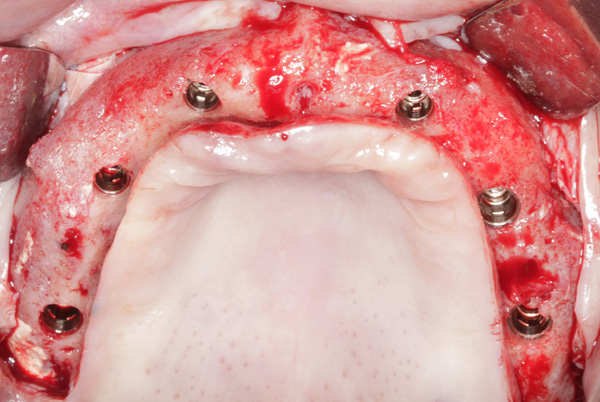 |
|
| Figure 23. Six dental implants were inserted into the reconstructed maxilla. |
Ideally, tension-free, primary flap closure is obtained over the graft site. Unlike non-absorbable membranes, exposure of conventional absorbable collagen membranes usually does not result in a serious infection. However, the bacterial contamination of the membrane results in faster degradation and may well compromise bone ingrowth. Studies have demonstrated greater resistance to breakdown by bacterial collagenases with ribose cross-linked collagen membranes after exposure to the oral cavity.13 A clinical study comparing native collagen, ePTFE, and ribose cross-link membranes found OSSIX Plus was capable of supporting gingival healing and bone fill even when prematurely exposed.14
CASE REPORTS
Case 1
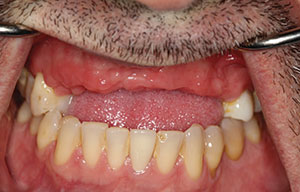
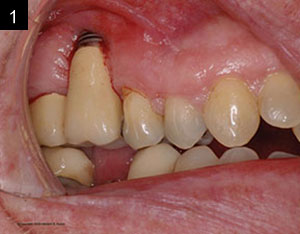
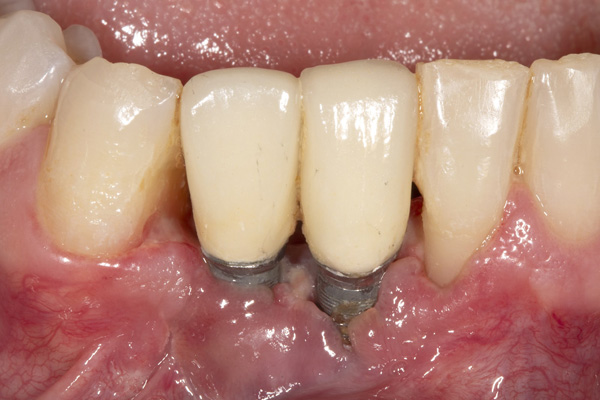
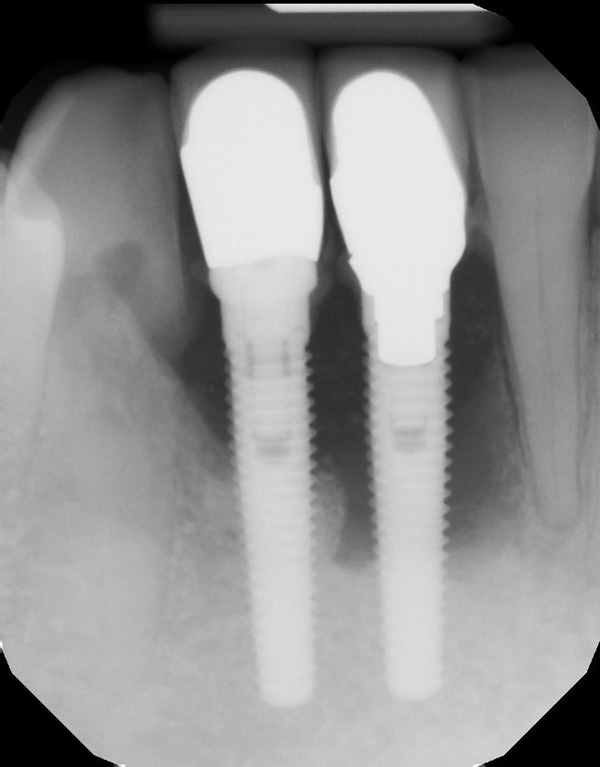
The patient was a 45-year-old male who had 2 failed implants in the anterior mandible (Nos. 25 and 26) (Figure 1). The periapical radiograph revealed severe bone loss around the implants and the adjacent teeth (Nos. 24 and 27) (Figure 2). The implants and the adjacent teeth were removed, and the area was allowed to heal for 2 months (Figure 3). The anterior mandible was planned for vertical bone augmentation for the placement of 2 implants to support an implant bridge. The ridge was exposed, and cortical perforations were performed in the graft site. A bone-harvesting bur (Auto-Max [Mega’Gen]) was used to procure particulate autograft from the mandibular symphysis (Figure 4). Tenting Screws (Pro-fix) were then inserted to provide added support for the particulate graft and space maintenance (Figure 5). The particulate autograft was mixed with bovine bone mineral (Bio-Oss [Geistlich Pharma]) and platelet-rich fibrin (IntraSpin System [BioHorizons]) (Figure 6). The graft was covered with an OSSIX Plus collagen membrane. Periosteal lasso sutures were then used to secure the membrane (Figure 7). The graft was then allowed to heal for 6 months (Figure 8). Re-entry found significant vertical bone gain and 3D reconstruction of the defect (Figures 9 and 10). Two Bone Level Tapered Implants (Straumann USA) were inserted into the anterior mandible (Nos. 25 and 27) (Figures 11 to 13).
Case 2
The patient was a 56-year-old female who presented with failing maxillary implants supporting a fixed bridge (Figure 14). Periapical radiographs revealed significant marginal bone loss around the implants (Figure 15). The implants were removed, and a temporary complete denture was delivered (Figure 16). Exposure of the maxilla revealed inadequate bone width for implant placement and a significant loss of facial contour (Figure 17).
Tenting screws were then inserted into the anterior maxilla (Figure 18). The particulate autograft was harvested with a bone scraper and mixed with bovine bone mineral (Bio-Oss) and platelet-rich fibrin (Intra-Spin System) (Figure 19). The graft was covered with OSSIX Plus collagen membranes. Periosteal lasso sutures were then used to secure the membranes (Figures 20 and 21). The graft was then allowed to heal for 6 months. Re-entry found significant vertical bone gain and 3D reconstruction of the defect (Figure 22). Six Straumann Bone Level Tapered Implants were inserted into the maxilla (Figure 23).
CONCLUSION
GBR has become a routine procedure in implant dentistry for predictable regeneration of bone and repair of osseous defects. Barrier membranes should be purposefully selected for each clinical scenario with consideration to their desired features. With several commercially available membranes, it is critical to be aware of their barrier function timeline, handling characteristics, and outcomes in clinical studies.
The OSSIX Plus collagen membrane is supported by evidence-based research and can offer excellent regenerative potential in demanding osseous defects due to its superior barrier function, degradation resistance, and ossification properties. The case examples in this article exhibit its use for horizontal and vertical bone augmentation using GBR with a resorbable membrane.
REFERENCES
1. Dahlin C, Linde A, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81(5):672–6. doi:10.1097/00006534-198805000-00004
2. Wang HL, Boyapati L. “PASS” principles for predictable bone regeneration. Implant Dent. 2006;15(1):8-17. doi:10.1097/01.id.0000204762.39826.0f
3. Simion M, Baldoni M, Rossi P, et al. A comparative study of the effectiveness of e-PTFE membranes with and without early exposure during the healing period. Int J Periodontics Restorative Dent. 1994;14(2):166-80.
4. Bunyaratavej P, Wang HL. Collagen membranes: a review. J Periodontol. 2001 Feb;72(2):215-29. doi:10.1902/jop.2001.72.2.215. https://pubmed.ncbi.nlm.nih.gov/11288796/
5. Moses O, Vitrial D, Aboodi G, et al. Biodegradation of three different collagen membranes in the rat calvarium: a comparative study. J Periodontol. 2008;79(5):905–11. doi:10.1902/jop.2008.070361
6. Rothamel D, Schwarz F, Sager M, et al. Biodegradation of differently cross-linked collagen membranes: an experimental study in the rat. Clin Oral Implants Res. 2005;16(3):369-78. doi: 10.1111/j.1600-0501.2005.01108.x
7. Tal H, Kozlovsky A, Artzi Z, et al. Long-term bio-degradation of cross-linked and non-cross-linked collagen barriers in human guided bone regeneration. Clin Oral Implants Res. 2008;19(3):295-302. doi:10.1111/j.1600-0501.2007.01424.x
8. Becker J, Al-Nawas B, Klein MO, et al. Use of a new cross-linked collagen membrane for the treatment of dehiscence-type defects at titanium implants: a prospective, randomized-controlled double-blinded clinical multicenter study. Clin Oral Implants Res. 2009;20(7):742-9. doi:10.1111/j.1600-0501.2008.01689.x
9. Scheyer ET, McGuire MK. Evaluation of premature membrane exposure and early healing in guided bone regeneration of peri-implant dehiscence and fenestration defects with a slowly resorbing porcine collagen ribose cross-linked membrane: a consecutive case series. Clin Adv Periodontics. 2015;5(3):165-170. doi:10.1902/cap.2014.130080
10. Misch CM, Basma H, Misch-Haring MA, Wang HL. An updated decision tree for vertical bone augmentation. Int J Periodontics Restorative Dent. 2021;41(1):11-21. doi:10.11607/prd.4996
11. Zubery Y, Nir E, Goldlust A. Ossification of a collagen membrane cross-linked by sugar: a human case series. J Periodontol. 2008;79(6):1101–7. doi:10.1902/jop.2008.070421
12. Friedmann A, Strietzel FP, Maretzki B, et al. Observations on a new collagen barrier membrane in 16 consecutively treated patients. Clinical and histological findings. J Periodontol. 2001;72(11):1616–23. doi:10.1902/jop.2001.72.11.1616
13. Klinger A, Asad R, Shapira L, et al. In vivo degradation of collagen barrier membranes exposed to the oral cavity. Clin Oral Implants Res. 2010;21(8):873–6. doi:10.1111/j.1600-0501.2010.01921.x
14. Moses O, Pitaru S, Artzi Z, et al. Healing of dehiscence-type defects in implants placed together with different barrier membranes: a comparative clinical study. Clin Oral Implants Res. 2005;16(2):210–9. doi:10.1111/j.1600-0501.2004.01100.x
ABOUT THE AUTHORS
Dr. Misch received his DDS degree from the University of Michigan in 1985. In 1991, he received certificates in postgraduate prosthodontics and oral implantology as well as a Master of Dental Science degree from the University of Pittsburgh. Dr. Misch then completed a residency in oral and maxillofacial surgery in Pittsburgh. He is board certified by the American Board of Oral and Maxillofacial Surgery as well as the American Board of Oral Implantology/Implant Dentistry. He is a Clinical Associate Professor at the University of Florida, University of Alabama, University of Pennsylvania, and University of Michigan. Dr. Misch serves as editor-in-chief of the International Journal of Oral Implantology and is on the editorial boards of several other dental journals. He practices as a dual specialist at Misch Implant Dentistry in Sarasota, Fla. He can be reached at cmisch@umich.edu.
Disclosure: Dr. Misch is a consultant for Datum Dental and Dentsply Sirona.
Dr. Misch-Haring is a third-year periodontics resident at the University of Alabama at Birmingham (UAB) School of Dentistry. She received her DMD degree from the UAB School of Dentistry in 2019, graduating as class valedictorian. Dr. Misch-Haring is a member of the Omicron Kappa Upsilon dental society, the American Academy of Periodontology, and the Academy of Osseointegration. She can be reached at mmisch@uab.edu.
Disclosure: Dr. Misch-Haring reports no disclosures.
RELATED ARTICLES
Impression Making of Implants Made Easy