|
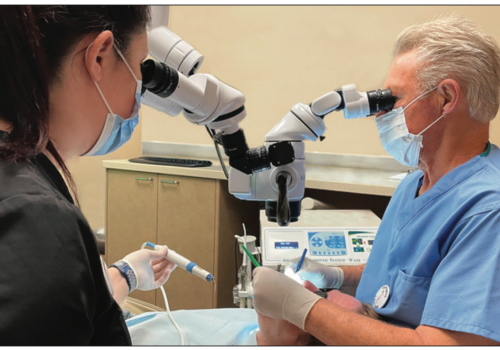
| Figure 1. Dental operatory with 80-in monitor, displaying 3-D camera image in a live demonstration. |
Dentistry is primarily a visual event for clinicians. After visually examining our patients’ teeth and gingiva, their radiographs, and watching a periodontal probe in sulci as it moves around their teeth, we are usually capable of diagnosing, treatment planning, and executing a restoration of their damaged hard and soft tissues. So any innovation that improves our ability to see what’s going on in our patients’ mouths, or to better see what we are doing procedurally, makes us more effective clinicians. In fact, it isn’t a far stretch to say that no other type of innovation has changed the practice of dentistry as much as imaging technology (Figure 1).
Of course, dental imaging includes both direct as well as indirect viewing paradigms—and, as such, dental radiography must be included in any article on this topic. This article will examine the past, present, and future of imaging in dentistry.
RADIOGRAPHY
Wilhelm Röntgen, a German physicist, discovered x-radiology in 1895 and for that discovery, received the first Nobel Prize in physics in 1901.1 His work resulted in unexpected outcomes. Dentists, not physicians, became the first to apply radiography to clinical practice. Saving teeth with infected root canal spaces (endodontic therapy) became a predictable dental procedure. With this imaging technology, endodontic procedures in the early 1900s came to be remarkably sophisticated as clinicians cut tapered root canal shapes, irrigated with sodium hypochlorite, and filled root canal spaces in 3 dimensions with chloro-percha obturation techniques. So, everybody was having a great time saving their patients’ pulpally diseased teeth until Walter Hess got a little too far ahead—conceptually—of the procedural power curve at the time.
VISUALIZING THE MICROANATOMY OF ROOT CANALS
In the book Hess et al2 published in 1925 on endodontic anatomy, the authors dramatically changed our understanding of the hidden spaces inside teeth. Hess et al accessed extracted teeth and dissolved the contents of their root canal spaces with the same solution (sodium hypochlorite) that is used to this day. Then, they injected vulcanite rubber into those spaces and dissolved the tooth structure away with nitric acid, leaving fantastically intricate models of the root canals. They also used chemicals to clarify dentin root structure of extracted teeth and then stained the root canal spaces with India ink, further demonstrating the incredible complexities of endodontic anatomy. Ironically, although this was a huge advance in our clinical understanding, it played a role in ending endodontics as a credible treatment regime in dentistry.
 |
 |
| Figure 2. (Left) Vulcanite rubber model by Hess et al2 (1925) of an upper molar with loop in its palatal canal and (right) 3-D computer reconstruction by Buchanan and Hebert (1989) of micro-CT imaging of an upper molar with loop in its palatal canal. |
Figure 3. (Left) Point cloud CT reconstruction of instrumentation results6 of Gates Glidden Burs and K-files versus GT Hand Files in extracted mesial root of a mandibular molar and (right) fully rendered reconstruction from another study* showing apical transportation caused by nonlanded shaping files (left canal) versus landed shaping files (right canal). *Chou, J, Buchanan, LS. A CT reconstructed comparison of shaping results in curved molar canals between landed and non-landed rotary files. Graduate research study, University of Southern California, 2004 (unpublished). |
 |
 |
| Figure 4. (Left) CT imaging of an upper molar with an obviously curved MB canal and (right) the same tooth with CT imaging, revealing a hidden severe apical bend of the DB canal. | Figure 5. (Left) Conventional posterior-anterior (PA) x-ray of upper molar with no obvious radicular pathosis and (right) CT image of the same molar showing extensive external resorption of its palatal root. |
 |
 |
| Figure 6. CT image showing hypertrophy of sinus membrane over untreated MB2 canal of upper molar. | Figure 7. (Left) Conventional x-ray showing no PA pathosis associated with lower cuspid and (right) CT image showing extensive mandibular osteonecrosis associated with the same cuspid tooth. |
 |
 |
| Figure 8. One of my cases where CT-based treatment planning was used for full-mouth placement of implant fixtures, with a panoramic radiograph showing the surgical result of implant placement. | Figure 9. CT-based treatment planning for Guided Endodontic Surgical (CT-GES) case. |
At the time this research was published, the Focal Infection Theory was in full rage (between 1920 and 1940). During this era, physicians were scapegoating dentistry for the disease states that they were not able to diagnose or cure, by claiming that treating teeth with root canal therapy (RCT) and a crown was the equivalent of creating “seas of sepsis underlying mausoleums of gold,” thus endangering their patients’ health.3 So at that time, finding out that root canals are more complex than most dentists realized was not good news. The profession lost heart that bacteria could be predictably eliminated in avascular spaces. For decades afterward, “extractionists” were lauded, and some dentists held that RCT should be criminalized and penalized with 6 months of hard labor. Truly, it was the dark age of modern dentistry.
Hess’ research was later reincarnated as the foundation for Dr. Herbert Schilder’s introduction of endodontic techniques designed to address the full apical and lateral complexity of root canals; an era that hit its stride just as I was heading into the specialty in the early 1980s. Being fascinated by the anatomic imperatives of procedural dentistry, I had a dream of recreating Hess’ anatomic studies using the new imaging technology that was coming out of Silicon Valley and, by chance, in 1989 I met my future research partner, Jean-Pierre Hebert, because he needed endodontic treatment.
On the wall of my operatory, I had a computer generated wire-frame model of root canal anatomy from an article I published in the California Dental Association Journal. Jean-Pierre asked me how it was created, why it interested me, and he then revealed that he was a fine artist in computer graphics.4 As we chatted, I mentioned that what I really wanted to do was to represent dental anatomy, not as an illustration like the Journal cover, but as it really existed in my patients’ teeth. He then told me that there was emerging computer imaging technology that could make that a possibility. Well, one thing led to another, and we found that General Electric (GE) had a micro-CT imaging center that they used to look for microfractures in the turbine fan blades of jet engines. We also found that there was 3-D reconstruction software that had just been developed by oil-hunting geologists. Furthermore, there was computer hardware invented by Silicon Graphics to run the software on that could render GE’s micro-CT imaging slices into virtual models of dental anatomy (Figure 2).
I got a loan for $200,000, bought a $125,000 Crimson VGX computer, loaded it with $60,000 worth of reconstructive software form Voxel View, and began scanning and rendering computer models of endodontic anatomy. We made animations of spinning transparent teeth, we looked at instrumentation results (Figure 3), and we made Hollywood-level animations of flying through root canal spaces.5,6 (Very expensive, but fun!) Something that stuck in my mind was how I never viewed the next tooth we reconstructed in virtual computer space (at that time it took 2 weeks to reconstruct a single tooth) without thinking, “What would it be like if we could see the anatomy in our patients’ teeth the same way we could see inside these extracted teeth we had scanned for 6 hours?” Who knew that would become commonplace in the clinical environment?
Operating Microscopes
For nearly 100 years, physicians have used operating microscopes—the first (1921) being Swedish otolaryngologist Carl-Olof Siggesson Nylén, considered the father of microsurgery. Later, in the 1950s, neurosurgeons began using operating microscopes; however, the most prestigious neurosurgical training centers denigrated the advantages brought by improved lighting and magnification, leaving it to outsiders such as Dr. Gazi Yaşarg to develop microneurosurgical techniques.7
Dentistry was a bit late to the microsurgical party until the early 1990s, when Dr. Gary Carr (an endodontist from San Diego) introduced operating microscopes and ultrasonic handpieces to the specialty of endodontics.8 Similar to the experience of medical microsurgeons, many of the prominent endodontists were skeptical. One of them was the head of a well-known university endodontic department, and later a president of the American Association of Endodontists (AAE), who expressed the opinion that the early adopting specialists were only using microscopes to justify higher fees.
Needless to say, he was proven dead wrong as microendodontic procedures swept the specialty as we used magnification, light, and ultrasonic cutting instruments to dramatically raise the bar and our success rates for retrograde endodontic surgical methods.9 After we incorporated microscopes and ultrasonic handpieces into our surgical practices, we realized that it was now possible to conventionally retreat failing root canals in ways that were impossible before. And again, our success rates for nonsurgical and surgical retreatment went up as we came to understand that many of our surgical failures were caused by placement of apical retro-seals against coronally leaking root canal fills.
 |
 |
| Figure 10. View of root apex in CT-GES case after apicesection with guided implant drills, ready for the retropreparation. | Figure 11. CT-based treatment planning for Guided Endodontic Access (CT-GEA) procedure and occlusal surface of molar after 0.8 mm CT-GEA cavities were laser cut. These openings led directly into the canals. |
 |
 |
| Figure 12. MoraVision 3-D clinical operating camera system. | Figure 13. The 3-D camera positioned over patient’s torso delivers a straight-on view of the occlusal surface of an upper molar without a mirror. |
With this kind of success story in endodontics, microscopes should logically have taken the rest of dentistry by storm, but that did not happen. To date, microscope adoption in general dentistry is less than 5%, and the adoption rate is even less in the specialties of oral surgery, periodontics, and implant placement.
Clinical CT Imaging
Fast forward from the early ’90s—when the only CT imaging available to dentistry was industrial scanning for anatomic representation and instrumentation research—to today when every one of my patients is CT scanned before diagnosis and treatment planning. What happened during the last 20 years?
The advance in technology that contributed most to this outcome was the development of CBCT imaging. The greatest advantage of CBCT imaging over conventional medical CT imaging is the unbelievably small amount of radiation absorbed by patients during a full-mouth imaging capture. The fan-beam medical CT scanning delivers 400 mSeverts of absorbed radiation versus the 25 to 40 mSeverts from CBCT scanning; less than an analog full-mouth series of conventional posterior-anterior (PA) radiographs. In addition, in only 7 to 9 seconds of scanning, CBCT machines image every part of a patient’s orofacial structures apical to the cemento-enamel junction, with no gagging, no retakes for cone cuts or missed radicular anatomy, and no anatomic artifacts.
Like everyone else who gets a CBCT machine, just as I experienced the introduction of operating microscopes to my practice, I began by using them “when needed.” So the list of indications began with all endodontic failures. This certainly included all cases to be retreated surgically, then teeth with possible root fractures were added, and then teeth that were diagnostically obscure, and so on. This proceeded until I had an epiphany related to a case I was planning to treat as a live demonstration for one of my hands-on training courses. All live demonstrations contain the possibility of a crash-and-burn situation, similar to NASCAR races. So, as one of the monthly courses we give at Dental Education Laboratories approached, I was faced with a truly heinous case to use, since the cases available for demos come randomly from my practice schedule. Looking at the PA radiographs of this upper molar with dilacerated curvature in all roots, I thought I wouldn’t normally “need” a CBCT for this case. However, if I’m risking professional humiliation in front of my students during a demo, it must be okay for me to capture a CT volume to lessen the possibility of being surprised by what I encounter after cutting an access cavity. What I found profoundly changed my practice experience (Figure 4). You see, in that particular case, I had found that the MB root had only a single canal (yes!), but I also found several severe canal curvatures hidden in the conventional PA radiographs; and this forewarning significantly reduced my chances of breaking a file in one of the canals. The next logical thought was whether it would help me diagnose and treat other cases that did not “need” CBCT imaging. What followed was a string of cases that changed my conceptual understanding and my practical decision making relative to CBCT imaging.
For a month, I had all patients scanned who were appointed for consultation. I found obscure but extensive resorptive defects (Figure 5); MB3 canals in upper molars and third mesial canals in lower molars; mid-root bifurcations of MB2 canals; soft-tissue anomalies of sinus membranes adjacent to endodontically failing root apices (Figure 6); spiral root fractures that couldn’t be found with a perio probe; and, most interesting, I found out how many periapical lesions do not show up on conventional radiographs (Figure 7).
I never really thought of it before using CBCT imaging, but periapical lesions can only be seen in conventional PA radiographs when bone surrounds the root apex. This obfuscation occurs more often than most of us realize, as many of our patients have had orthodontic treatment without first premolar extraction, palatal expansion, or orthognathic surgery. The result is a beautiful and broad arch of teeth but can result in a remarkable number of root apices being outside the buccal cortical plate, therefore presenting the possibility of periapical pathosis without radiographic lucency. Beyond these challenges to clinical visualization, CBCT imaging overcomes anatomic artifacts (such as the obscuration of orofacial structure by natural anatomic objects in our patients’ jaws), other roots, malar eminences, etc.
My good friend Dr. Roger Warren (Salt Lake City, Utah) explained to me that his CBCT machine pays for itself every month just from the preoperative identification of hopeless teeth that would have previously required exploratory access and a wasted appointment time before determining they had poor prognoses. Similar to his experience, he said, hospitals used to have a stable of surgeons on hand to do exploratory surgery when patients arrived with unknowable disease states. Now with CT and MRI imaging, surgery is nearly always done only after definitive diagnosis has been accomplished, dramatically reducing morbidity and mortality.
Beyond diagnosis and conventional treatment planning, CBCT imaging has seriously changed our clinical outcomes with the advent of CT-guided procedures. While CT-guided implant surgery has been done for more than a decade (Figure 8), this same elegant treatment planning modality is now being developed as a treatment planning tool and procedural aid in endodontics. In 2013, I did the first live demonstration of CT-Guided Endodontic Surgery (CT-GES), using SimPlant (Dentsply Sirona Implants) treatment planning software, at the AAE meeting in San Diego (Figure 9).
Was this just showing off, or were these methods that would advance clinical endodontics? If we look at the challenge of training dentists to do apical surgery, as well as the time and difficulty of performing those procedures even when clinicians are fully competent, it is not a far stretch to see the advantages gained with securing a CT-based surgical guide to a patient’s teeth, making a single incision that is 5.0 mm in length (far away from attached gingiva), then using 2 or 3 drills through the guide to a predetermined length, resulting in a perfectly resected root apex ready for the retrofill to follow (Figure 10). In this scenario, it is conceivable that an apical procedure could be done on a palatal root of an upper molar in less than 20 minutes.
CT-Guided Endodontic Access (CT-GEA)
It is becoming better understood that the long-term prognosis of an endodontically treated tooth is more greatly influenced by its structural integrity after RCT access and shaping procedures than any other factor, as current technology allows resolution of virtually every endodontic disease state. Smaller access cavities and less coronal shaping will undoubtedly result in longer-term treatment outcomes—the only credible endodontic answer to the excellent prognoses delivered by implant replacement when the structural integrity of a previously treated tooth is upside down.
At the ADA meeting this past November, I did the first live demonstration of CT-GEA, using an Er:YAG laser (AdvErL Evo Er:YAG Laser [J. Morita USA]) to cut an entry hole just 0.08 mm in diameter, after which a No. 15 K-file dropped through, perfectly entering the DB canal, resulting in a tooth saved with literally no loss of structural integrity (Figure 11).
Just like all new technology, those highly skilled in the art are least likely to see the advantages of doing things in new improved ways as expert clinicians have already learned to deal with the challenges presented with existing procedures. So it is that procedural advances are often taken up by novice clinicians who, having less experience and are less invested in prior training, can more easily appreciate the advantages of having entire procedures worked out before a single cutting tool is applied to a patient’s body. With that said, it seems to me to be a foregone conclusion that CT-GES and CT-GEA will become a valued part of endodontic treatment modalities, just as CT-guided surgery has become the gold standard of implant placement.
3-D Magnification
That brings us back to magnification and light for direct visualization of procedures. Where will technology take us to improve what we have now with operating microscopes? The best answer I have seen is now ready for prime time after 10 years of development by Dr. Assad Mora, a Santa Barbara prosthodontist.
There are several reasons that microscopes have not become popular throughout the rest of dentistry while they have become a necessity in endodontics. First off, endodontics turns out to be the most elegant procedure with which to apply operating microscopes. This is because every endodontic procedure, both conventional and surgical, is done through small openings in teeth and through soft and hard tissues. This makes the primary limitations of operating microscopes relatively moot (with their shallow depths of focused field and their remarkably limited range of positioning) as clinicians must be able to place their eyes on the binocular eyepieces after the microscope has been situated over the operating field. Secondary limitations are the challenges of suspending the heavy optical elements from a wall or ceiling.
Dr. Mora has ingeniously designed a 3-D camera system that solves all of these problems. His 3-D System (MoraVision) consists of a 4-in cube housing 2 high-definition video cameras, a light source, a microphone, and a 3-D video microprocessor, all suspended by a relatively small articulating arm on a pole by the patient’s hip (Figure 12). One of the many inventions Dr. Mora has included in his camera setup is a flat aluminum paddle below the supporting pole, allowing the camera to be installed in an operatory just by lifting the dental chair and slipping the thin paddle underneath, securing the support structure with the weight of the chair. (That’s clever!) The remainder of installation involves plugging in the AC power cord, the HDMI cable to a nearby 3-D monitor, and positioning the foot control next to the handpiece foot control. That’s it. No contractor, no tech people needed. The magnified 3-D image is viewed in real time with passive 3-D glasses; this type uses 2 polarizing filters, one set on either side of the glasses frame at 90° to one another. No batteries are needed, and the glasses cost less than $5 each. The degree of magnification varies by the size of the viewing monitor, but with a 42-in monitor it delivers somewhere between 10x and 13x. With a larger monitor (a 79-in LG 3-D screen) the magnification is greater than 16x—fully competitive with the Zeiss ProErgo that I replaced.
This camera has a magnificent one-in to 3-in depth of field versus the one eighth-inch to half-inch depth of field that microscopes offer, allowing clinicians to see anterior and posterior teeth in focus simultaneously at 8x; this is an impossibility with microscopes. It is my opinion that depth-of-field issues were the greatest limitations of microscopes for use in general dentistry, the requirement being much greater than the tiny operative field worked through in endodontic procedures.
The second greatest limitation of microscopes in general dentistry has been the extremely limited 15° range of position allowed by microscopes because of the posture-dependent requirement that clinician’s eyes meet the eyepieces to view the operative field. With MoraVision, I can lean in, lean back, and move from side to side without moving the camera while viewing the operative field up on the screen rather than down toward patient’s mouth. In other words, I can virtually position my eyes independent of the image capture device (the camera), independent of my posture. Being a typical dentist with back problems between the L4 and 5 vertebrae, my post-procedure fatigue and back soreness is a small fraction of what I experience after holding a static position for the 10-minute blocks of time in a row required with eyepiece viewing. Beyond issues of the clinician’s body positioning, the wide range of camera positioning allows me to cut access cavities in maxillary teeth without a mirror (now that’s a trip!), and to even place the camera to the side of the patient’s mouth when I want to view a file stop meet its reference point without parallax error (Figure 13).
While several microscope companies offer 3-D visualization on 3-D TV monitors through video cameras placed on either side of the beam splitter, they typically cost upwards of $100,000. MoraVision is half the cost of a new ProErgo, and I can automatically feed 2-D image captures into my chairside electronic dental charting system as well as record 2-D and 3-D video to my chairside computer hard drive or my MP4 compression digital video recorder. Finally, the learning curve to competence in the use of this new imaging system (MoraVision) is a fraction of that required to learn how to use loupes because of its remarkably wide field of view, and also that of microscopes because of its limitless range of camera positioning. The thought of looking at a TV monitor instead of looking directly into the patient’s mouth seems like it would be a significant impediment to early use; however, the beautiful projection of the third dimension through the monitor makes it a very intuitive experience; one that most catch onto in less than about 10 minutes of use.
This is undoubtedly a new inflection point in dental imaging.
CLOSING COMMENTS
Without doubt, it will be difficult for microscope users to consider replacing them with a 3-D camera. That is how all new technology is perceived by those who have worked hard and learned to do things the more difficult way, with old technology. For those younger dentists who have never used a microscope, and those older microscope-using dentists with back and neck issues, using a 3-D clinical camera will be the only game in town.
Remember the 3 stages of innovation acceptance: first, “That’s ridiculous!”; then “OK, it’s not ridiculous, but I don’t need it”; and then “How did we ever do this before?”
References
- Roentgen WC. Fundamental contributions to the X-ray: the three original communications on a new kind of ray. Translated from reprints of the original papers (1895-1897) by Ernest Kraft, 1972. Located at: US National Library of Medicine, Bethesda, MD.
- Hess W, Zürcher E, Dolamore WH. The Anatomy of the Root-Canals of the Teeth of the Permanent Dentition. New York, NY: William Wood & Company; 1925: 199.
- Hunter W. Oral sepsis as a cause of disease. Br Med J. 1900;2:215-216.
- Buchanan LS. Management of the curved root canal. J Calif Dent Assoc. 1989;17:18-27.
- Buchanan LS. Endodontics in the fourth dimension: dentin meets the silicon chip in new millennium. Dent Today. 1995;14:82-87.
- Gluskin AH, Brown DC, Buchanan LS. A reconstructed computerized tomographic comparison of Ni-Ti rotary GT files versus traditional instruments in canals shaped by novice operators. Int Endod J. 2001;34:476-484.
- Yaşargil MG. Microsurgery: Applied to Neurosurgery. Stuttgart, Germany: Georg Thieme Verlag; 1969.
- Carr GB. Microscopes in endodontics. J Calif Dent Assoc. 1992;20:55-61.9
- Carr GB. Ultrasonic root end preparation. Dent Clin North Am. 1997;41:541-554.
Dr. Buchanan is a Diplomate of the American Board of Endodontics, a Fellow of the National and International Colleges of Dentists, and part-time faculty at University of California, Los Angeles’ and University of Southern California’s graduate endodontic programs. He is the founder of Dental Education Laboratories, a hands-on teaching center in Santa Barbara, where he also maintains a practice limited to conventional/microsurgical endodontic therapy and implant surgery. He can be reached via the websites delendo.com and endobuchanan.com.
Disclosure: Dr. Buchanan discloses a financial interest in the following products: TrueTooth (DELendo), System B/Elements Obturation Unit, Buchanan Pluggers/Endobender Pliers, GTX Files and associated products, GT Series Files and associated products, BUC Access Refinement Tips, LA Axxess Burs, and Root ZX Mini (DENTSPLY Tulsa Dental Specialties).