|
INTRODUCTION
Surgical approaches to the management of obstructive sleep apnea (OSA) include uvulopalatopharyngoplasty (UPPP) and similar procedures. For example, Pang and Woodson1 assessed the efficacy of expansion sphincter pharyngoplasty (ESP) in the treatment of OSA. They reported that the apnea-hypopnea index (AHI) decreased from 44 to 12 events/hour following ESP and from 38 to 19 events/hour in a similar group that had UPPP. But, it appears that these surgical techniques were unable to eradicate OSA entirely. Similarly, Bertoletti et al2 found a significant reduction in continuous positive airway pressure (CPAP) levels 3 months after minimally invasive pillar placements, but the underlying OSA remained unresolved. On the other hand, Conley and Legan3 demonstrated the importance of surgical transverse expansion of the maxillary and mandibular arches in patients with severe OSA. They found a marked improvement in the degree of OSA, but the severity of the surgical procedure, which included distraction osteogenesis, cannot be overlooked. Similarly, Bonetti et al4 reported a case of severe OSA in which an increase in the transverse dimensions by surgically-assisted rapid maxillary expansion and mandibular symphyseal distraction osteogenesis dramatically decreased the AHI to 9 events/ hour. Therefore, it appears that surgical techniques are able to moderate but not eliminate OSA entirely.
A possible alternative to surgical intervention might be nonsurgical manipulation of the upper airway. In a recent study,5 the upper airway was evaluated following midfacial treatment in adults who had never had been tested for OSA. Pre- and post-treatment lateral cephalographs of 99 patients (mean age 42.9 ± 1.5 years: treatment time 21.3 ± 6.2 months) were analyzed using appropriate software. Using 2-dimensional finite-element analysis, a relative 22% increase in nasopharyngeal airway area was found above and behind the soft palate.6 It was concluded that upper airway changes in nongrowing adults are similar to those of actively growing children undergoing functional orthodontic corrections,7 and these findings suggested that genetically-encoded developmental mechanisms might be modulated by maxillary appliances to enhance the upper airway in adults. A new case is described here to substantiate these implications in the management of adults diagnosed with OSA.
|
CASE REPORT
Diagnosis and Treatment Planning
A 36-year-old male diagnosed with OSA following overnight polysomnography (PSG) presented, seeking a nonsurgical alternative to bimaxillary advancement for the correction of his condition. The PSG report indicated an AHI of 24 events/hour and a mean oxyhemoglobin saturation of 90%. He was on CPAP therapy at an average pressure of 16.5 cm H2O and was taking 30 mg mirtazapin (Remeron), 500 mcg melatonin, and one mg klonopin, as needed, which was almost every night.
Upon intraoral examination, his maxillary minimum intermolar width was recorded as 34 mm at the cervical margin of the mesiopalatal cusps of the first permanent molars. His maxillary and mandibular first premolars were missing, and the patient volunteered that these had been extracted as part of an earlier orthodontic procedure. Diagnostic records were taken, including facial photographs, a 3-dimensional cone-beam computed tomography scan (iCAT [Imaging Sciences]), as well as impressions for study models. His mandibular trajectory was recorded using a jaw-tracking device (Myotronics). All treatment procedures were carried out by Dr. Wendling.
Treatment
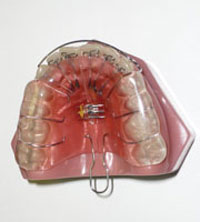
After reaching a differential diagnosis of midfacial underdevelopment, the treatment objectives were listed as follows: to increase the maxillary transverse direction, to reposition the mandible anteriorly, and to reduce the AHI to within normal limits. A customized maxillary appliance (Figure 1) was fabricated (as well as a mandibular appliance). After insertion, the patient was instructed to wear the maxillary appliance during the evening and every night for at least 12 to 16 hours, and to return for periodic adjustments every 3 to 4 weeks. In the interim, the patient was instructed to turn the appliance’s midline expansion screw when the appliance became loose. After 10 months, the patient was studied using type IV monitoring device (ApneaLink [ResMed Corp]).
Outcome
The patient reported that he wore the maxillary appliance each evening and every night for an average of approximately 16 hours. The patient did not wear the appliance for approximately 8 hours during the day. The midline expansion screw was advanced approximately twice a week (0.25 mm on each turn). The patient reported that he gradually reduced the CPAP pressure by about 70% as the treatment proceeded. He also reduced the dosage of Remeron from 30 mg to 7.5 mg. By 10 months of treatment, the patient reported that he no longer took any klonopin but continued with melatonin at a dose of 250 mcg because he believes he is sensitive to changes in his sleep schedule.
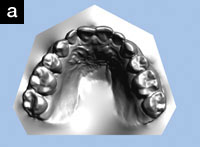
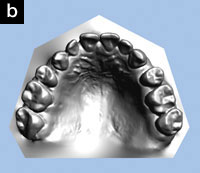
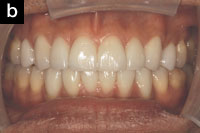
Upon intraoral examination, the minimum intermolar width had increased from 34 mm to 39 mm (Figures 2a and 2b). The patient reported that the development of his upper arch seemed to have helped him breathe through his nose. In addition, the mandible repositioned itself further forward by 2.9 mm with the mandibular appliance, according to the jaw-tracking data (Figures 3a and 3b).
Cone Beam Computed Tomography Results
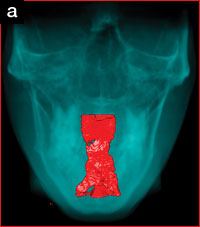
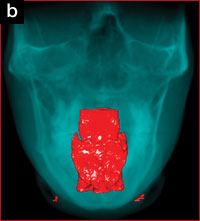
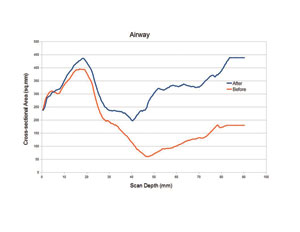
The patient’s upper airway was segmented and reconstructed from the level of the posterior nasal apertures to the level of the hyoid bone, using appropriate software. The upper airway volume increased by 58% from 12,889 mm3 to 22,024 mm3 (Figures 4a and 4b), and the minimum cross-sectional area showed corresponding increases (Figure 5).
Sleep Architecture
The patient was studied using a type IV monitoring device from 22:00 pm to 04:58 am for a flow evaluation period of 2 hours and 24 minutes and SpO2 evaluation period of 6 hours and 37 minutes. The patient had an AHI of zero events/ hour. The oxygen desaturation index was 95%. The lowest oxygen saturation was 88%. Approximately 3 minutes were spent with an oxygen desaturation between 85% to 90%. Therefore, some flow limitation was noted, although this was within normal parameters. Extended pulse, oxygen saturation, and limb movement profile were not available. However, the cardiac profile showed a minimum pulse frequency of 47 beats per minute, while the maximum pulse frequency was 161 beats per minute, with an average pulse frequency of 63 beats per minute.
DISCUSSION
Current, nonsurgical, dental management of OSA typically deploys the use of mandibular advancement devices. However, side effects associated with this modality include: unwanted tooth movements8; the precipitation of anterior crossbite,9 and temporomandibular changes in some cases.10 Therefore, maxillary appliances that putatively induce renewed midfacial development might provide an alternative approach, by permitting nonsurgical remodeling (pneumopedics) of the upper airway. A type IV monitoring device was used to detect changes in respiratory events such as apneas and hypopneas post-treatment. Using this device, the evidence for sleep disordered breathing appears to be within a normal range for this particular case study. However, the scored AHI may possibly underestimate the severity of sleep-disordered breathing in this patient as other history and physical details were not available. Therefore, further evaluation and treatment should be discussed with the patient because a normal ApneaLink study does not rule out sleep disordered breathing, and a full diagnostic PSG should be obtained if clinically indicated. However, the ApneaLink is an ambulatory sleep monitor that can detect OSA and/or hypopnea with acceptable reliability.11
A recent study suggests that both the maxillary and the mandibular dental arches are smaller in adults with OSA.12 Therefore, maxillary appliances that putatively induce midfacial development might provide an alternative approach, by permitting nonsurgical (pneumopedic) remodeling of the upper airway. According to Stellzig-Eisenhauer and Meyer-Marcotty,13 it is not disputed that expanding the maxilla improves not only nasal volume and nasal flow, but also the subjective sensation of patients, and this seems to be our experience in this particular case. Indeed, in the opinion of Posnick and Agnihotri,14 in patients with a dentofacial deformity, treatment of the maxillary dysplasia with a specific emphasis on transverse expansion appears to address the chronic difficulties with nasal breathing, inter alia. Therefore, in patients with normal craniofacial architecture, one might anticipate a similar outcome, and an improvement in nasal breathing appears to be the case in this particular patient also. In support of these findings, Rose and Schessl15 reported that enlarging the upper airway and preventing its collapse using orthodontic means in children with craniofacial anomalies serves to successfully treat OSA. However, the response of children with no craniofacial abnormalities remains unaddressed. In this regard, Villa et al16 assessed rapid maxillary expansion (RME) in the treatment of OSA in children devoid of other craniofacial abnormalities. Polysomnography showed a significant decrease in the AHI, and the authors concluded that RME is an effective appliance for treating children with OSA. Similarly, Pirelli et al17 investigated whether RME could improve the patency of the nasal airways and OSA. They reported that RME therapy widens the nasal fossa, releases the nasal septum, restores nasal airflow, and that these functional improvements are associated with a reduction of sleep disordered breathing in children. In addition, Miano et al18 also evaluated REM sleep microstructure in children with OSA before and after one year of RME treatment. They also found that after one year of treatment, the AHI in children with OSA decreased significantly compared to controls. Therefore, RME appears to improve sleep architecture in children. We believe that the adult case reported here showed a similar response.
CONCLUSION
Maxillary appliances that putatively induce renewed midfacial development might provide an alternative approach to managing OSA, by permitting nonsurgical remodeling of the upper airway. However, a normal ambulatory sleep study does not rule out sleep disordered breathing or upper airway resistance, and the scored AHI may underestimate the current severity of OSA in this patient and further follow-up to obtain a full diagnostic PSG is warranted. Nevertheless, the facial and dental outcomes for this particular patient (Figures 6a and 6b) seem to provide further support for the protocol described here.
Acknowledgements
The authors would like to thank Quint Whipple, Phoenician Dental Studios for the cosmetic veneers.
References
- Pang KP, Woodson BT. Expansion sphincter pharyngoplasty: a new technique for the treatment of obstructive sleep apnea. Otolaryngol Head Neck Surg. 2007;137:110-114.
- Bertoletti F, Indelicato A, Banfi P, et al. Sleep apnoea/hypopnoea syndrome: combination therapy with the Pillar palatal implant technique and continuous positive airway pressure (CPAP). A preliminary report. B-ENT. 2009;5:251-257.
- Conley RS, Legan HL. Correction of severe obstructive sleep apnea with bimaxillary transverse distraction osteogenesis and maxillomandibular advancement. Am J Orthod Dentofacial Orthop. 2006;129:283-292.
- Bonetti GA, Piccin O, Lancellotti L, et al. A case report on the efficacy of transverse expansion in severe obstructive sleep apnea syndrome. Sleep Breath. 2009;13:93-96.
- Singh GD, Krumholtz JA. Epigenetic Orthodontics in Adults. Chatsworth, CA: SMILE Foundation; 2009.
- Singh GD, McNamara JA Jr, Lozanoff S. Morphometry of the cranial base in subjects with Class III malocclusion. J Dent Res. 1997;76:694-703.
- Singh GD, Garcia-Motta AV, Hang WM. Evaluation of the posterior airway space following Biobloc therapy: geometric morphometrics. Cranio. 2007;25:84-89.
- Ueda H, Almeida FR, Lowe AA, et al. Changes in occlusal contact area during oral appliance therapy assessed on study models. Angle Orthod. 2008;78:866-872.
- Korngut R. Case study: A persistent positive outcome treating OSA with a MAD following the treatment protocol. Dialogue. 2010;1:26-27.
- Almeida FR, Lowe AA, Sung JO, et al. Long-term sequellae of oral appliance therapy in obstructive sleep apnea patients: Part 1. Cephalometric analysis. Am J Orthod Dentofacial Orthop. 2006;129:195-204.
- Chen H, Lowe AA, Bai Y, et al. Evaluation of a portable recording device (ApneaLink) for case selection of obstructive sleep apnea. Sleep Breath. 2009;13:213-219.
- Banabilh SM, Suzina AH, Dinsuhaimi S, et al. Dental arch morphology in south-east Asian adults with obstructive sleep apnoea: geometric morphometrics. J Oral Rehabil. 2009;36:184-192.
- Stellzig-Eisenhauer A, Meyer-Marcotty P. Interaction between otorhinolaryngology and orthodontics: correlation between the nasopharyngeal airway and the craniofacial complex [in German]. Laryngorhinootologie. 2010;89(suppl 1):S72-S78.
- Posnick JC, Agnihotri N. Consequences and management of nasal airway obstruction in the dentofacial deformity patient. Curr Opin Otolaryngol Head Neck Surg. 2010;18:323-331.
- Rose E, Schessl J. Orthodontic procedures in the treatment of obstructive sleep apnea in children [in English, German]. J Orofac Orthop. 2006;67:58-67.
- Villa MP, Malagola C, Pagani J, et al. Rapid maxillary expansion in children with obstructive sleep apnea syndrome: 12-month follow-up. Sleep Med. 2007;8:128-134.
- Pirelli P, Saponara M, Attanasio G. Obstructive Sleep Apnoea Syndrome (OSAS) and rhino-tubaric disfunction in children: therapeutic effects of RME therapy [in English, Italian]. Prog Orthod. 2005;6:48-61.
- Miano S, Rizzoli A, Evangelisti M, et al. NREM sleep instability changes following rapid maxillary expansion in children with obstructive apnea sleep syndrome. Sleep Med. 2009;10:471-478.
Dr. Singh is currently president of BioModeling Solutions, director of continuing education for the SMILE Foundation, senior instructor/consultant/Fellow for the International Association for Orthodontics, academic Fellow of the World Federation of Orthodontists, and chair of research for the Academy of Sports Dentistry. Previously, he was adjunct professor at Portland State University, visiting professor in Orthodontics (Malaysia), and associate professor at the University of Puerto Rico. He has been published extensively in orthodontic and dental literature, and is co-author of the book entitled Epigenetic Orthodontics in Adults. Dr Singh has lectured internationally. He holds several US patents with international patents pending. He can be reached at (503) 430-7529/7536 or at drsingh@drdavesingh.com.
Disclosure: Dr. Singh is president of BioModeling Solutions, LLC, and holds all patents for the DNA Appliance System.
Dr. Wendling graduated at Oregon Health & Sciences University School of Dentistry, Portland, Ore. She attended Portland State University for her predental studies. Prior to that, she graduated from West Liberty State College, West Virginia School of Dental Hygiene with an associate in science in dental hygiene. She also attended Eastern Kentucky University, Richmond, Ky, for her predental hygiene requirements. Dr. Wendling’s current professional affiliations include: the American Academy of Dental Sleep Medicine, the AGD (Fellow), the American Academy of Cosmetic Dentistry (active member), and the International Congress of Cranio Mandibular Orthopedics. She can be reached at (503) 636-4069 or at drsue@drwendling.com.
Disclosure: Dr. Wendling reports no disclosures.
Dr. Chandrashekhar was born in Bangalore, India, and has been residing in the United States since early childhood. He attended the State University of New York at Buffalo where he received a BS in electrical engineering and a minor in mathematics. He worked in industry at NASA in Houston, Tex, as well as Alcatel Telecom. Dr. Chandrashekhar earned a MS in electrical engineering at the University of Texas at Dallas. Upon completion of his internal medicine residency, he went straight into a competitive sleep medicine Fellowship at the New Jersey Neuroscience Institute at the JFK Medical Center in Edison, NJ. He can be reached at (760) 843-0100 or via e-mail at rc@eusomnia.com.
Disclosure: Dr. Chandrashekhar reports no disclosures.
{rscomments on}