The decay-causing bacteria that live in dental plaque “hide” in a glue-like polymer scaffold. Embedded in this sticky biofilm matrix, this bacteria then can resist traditional antimicrobial treatment. Researchers at the University of Pennsylvania, though, have developed a new strategy to combat these unwelcome invaders.
Instead of applying an antimicrobial to the teeth, the researchers used the pH-sensitive and enzyme-like properties of iron-containing nanoparticles to catalyze the activity of hydrogen peroxide. The activated hydrogen peroxide produced free radicals that simultaneously degraded the biofilm matrix and killed the bacteria within, significantly reducing plaque and preventing tooth decay in an animal model.
“Even using a very low concentration of hydrogen peroxide, the process was incredibly effective at disrupting the biofilm,” said Hyun (Michael) Koo, professor in the Penn School of Dental Medicine’s department of orthodontics and divisions of pediatric dentistry and community oral health, as well as senior author of the study. “Adding nanoparticles increased the efficiency of bacterial killing more than 5,000-fold.”
Previous work had shown that nanoparticles, long believed to be biologically and chemically inert, could possess enzyme-like properties. It also showed that an iron oxide nanoparticle behaved similarly to a peroxidase, an enzyme found naturally that catalyzes oxidative reactions, often using hydrogen peroxide.
In 2013, postdoctoral researcher Lizeng Gao proposed using these nanoparticles in an oral setting since the oxidation of hydrogen peroxide produces free radicals that can kill bacteria.
“When he first presented it to me, I was very skeptical,” Koo said, “because these free radicals can also damage healthy tissue. But then he refuted that and told me this is different because the nanoparticles’ activity is dependent on pH.”
Gao, lead author of the study, found that the nanoparticles had no catalytic activity at neutral or near-neutral pH of 6.5 or 7, physiological values typically found in blood or a healthy mouth. But when pH was acidic, closer to 5, they became highly active and could produce free radicals.
The scenario was ideal for targeting plaque, which can produce an acidic microenvironment when exposed to sugars. The researchers then set out to synthesize, characterize, and test the effectiveness of the nanoparticles, several forms of which are already approved by the US Food and Drug Administration for imaging in humans.
Beginning with in vitro studies, which involved growing a biofilm containing Streptococcus mutans on a tooth-enamel-like surface and then exposing it to sugar, the researchers confirmed that the nanoparticles adhered to the biofilm, were retained even after treatment stopped, and could effectively catalyze hydrogen peroxide in acidic conditions.
Also, the researchers showed that the nanoparticles’ reaction with a 1% or less hydrogen peroxide solution was remarkably effective at killing bacteria, wiping out more than 99.9% of the S mutans in the biofilm within 5 minutes, which is more than 5,000 times more effective than using hydrogen peroxide alone.
Even more promising, they demonstrated that the treatment regimen, involving a 30-second topical treatment of the nanoparticles followed by a 30-second treatment with hydrogen peroxide, could break down the biofilm matrix components and essentially remove the protective sticky scaffold.
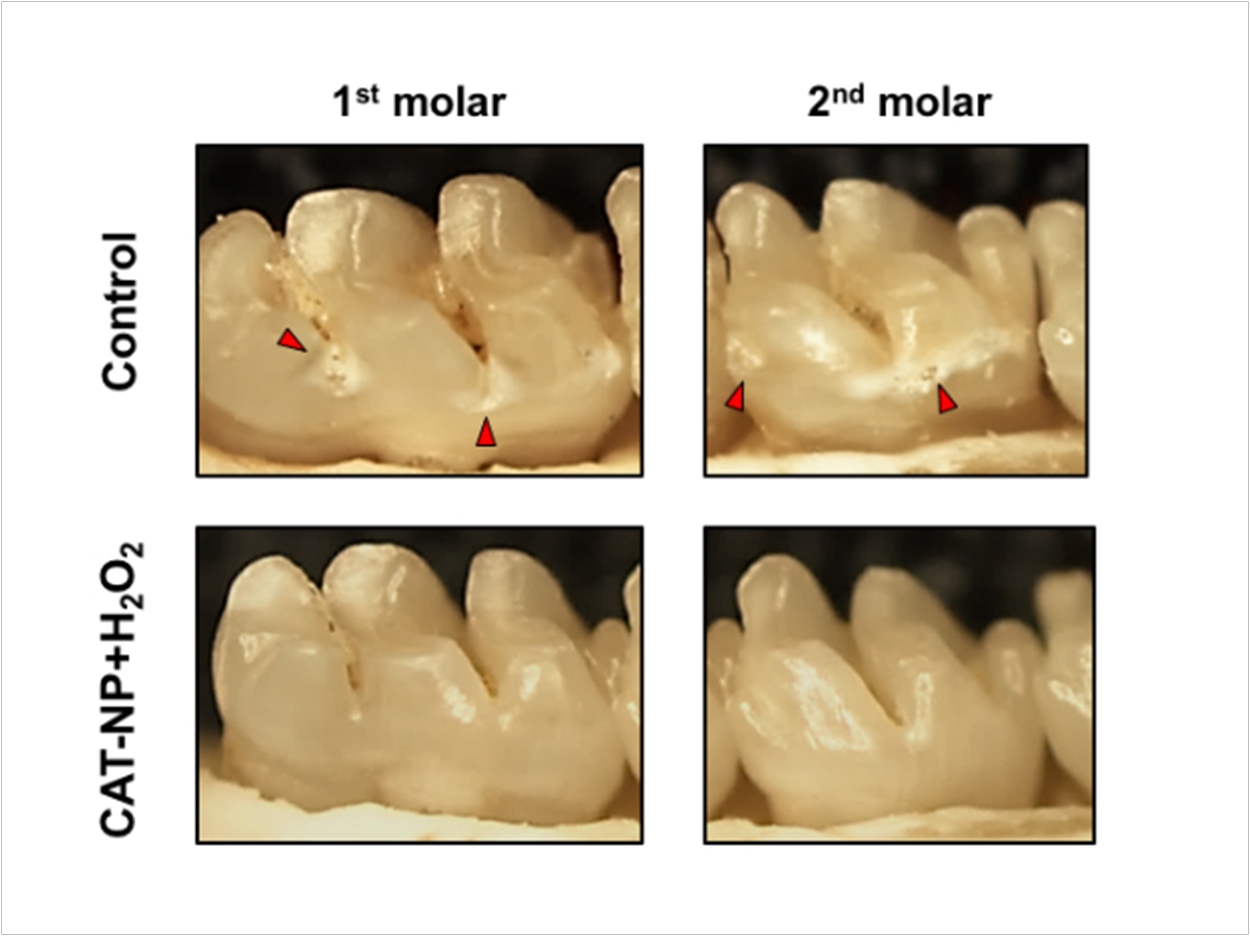
Moving to an animal model, the researchers applied the nanoparticles and hydrogen peroxide topically to the teeth of rats. Three weeks of twice-a-day, one-minute treatments significantly reduced the onset and severity of carious lesions, compared to the control or treatment with hydrogen peroxide alone. There were no adverse effects on the gum or oral soft tissues from the treatment either.
“It’s very promising,” said Koo. “The efficacy and toxicity need to be validated in clinical studies, but I think the potential is there.”
“If you look at the amount you would need for a dose, you’re looking at something like 5 mg,” said David Cormode, coauthor of the study and an assistant professor of radiology and bioengineering with appointments at the Penn Perelman School of Medicine and School of Engineering and Applied Science. “It’s a tiny amount of material, and the nanoparticles are fairly easy to synthesize, so we’re talking about a cost of cents per dose.”
Also, the platform’s 1% concentration of hydrogen peroxide is lower than many currently available tooth-whitening systems that use 3% to 10% concentrations, minimizing the chance of side effects. Next, the researchers hope to continue refining and improving upon the effectiveness of the nanoparticle platform to fight biofilms.
“We’re studying the role of nanoparticle coatings, composition, size, and so forth so we can engineer the particles for even better performance,” said Cormode.
The study, “Nanocatalysts Promote Streptococcus Mutans Biofilm Matrix Degradation and Enhance Bacterial Killing to Suppress Dental Caries In Vivo,” was published by Biomaterals.
Related Articles
Imaging Reveals Structure Behind Dentin’s Durability
Silver Enlisted to Stop Caries and Extend Filling Lifespans
S Mutans Sticks to Ceramics, Too












