Researchers at the University of California Los Angeles (UCLA) have developed methods that may lead to effective and reliable therapy for periodontal disease that promotes gum tissue and bone regeneration with biological and mechanical features that can be adjusted based on treatment needs.
Current treatment for periodontitis includes infection-fighting methods, the application of molecules known as growth factors that promote tissue growth, and guided tissue regeneration, which is considered the optimal standard of care.
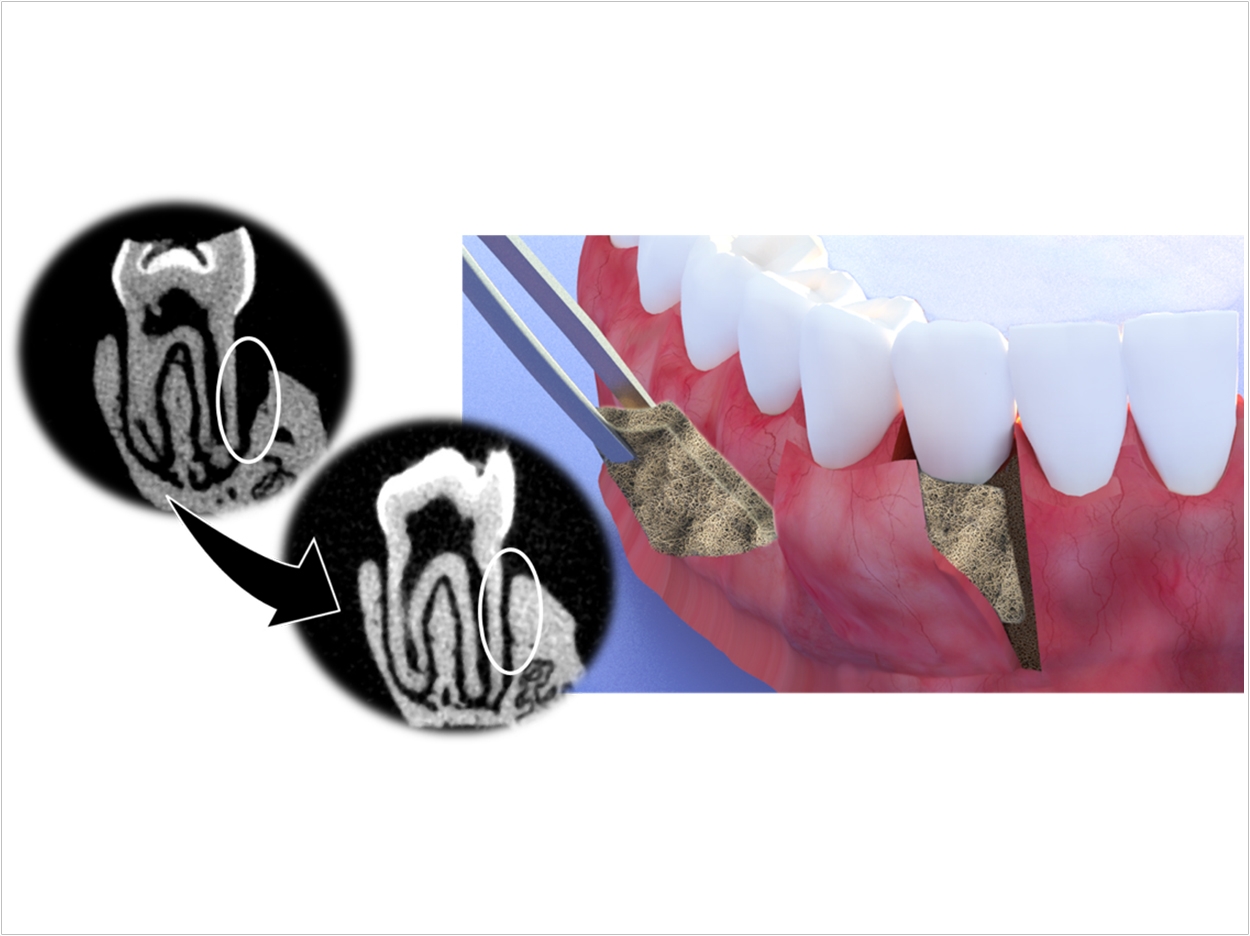
In guided tissue regeneration, a membrane or thin film is surgically placed between the inflamed gum and the tooth. Membranes, which come in biodegradable and non-biodegradable forms, are meant to act as barriers between the infection and the gums as well as a delivery system for drugs, antibiotics, and growth factors to the gum tissue.
Guided tissue regeneration results are inconsistent, though. Current membranes are unable to regenerate gum tissue directly or maintain their structure and stability when placed in the mouth. The membrane also can’t support prolonged drug delivery, which is necessary to help heal infected gum tissue. Plus, non-biodegradable membranes require multiple surgeries for removal after drugs have been released, compromising healing.
“Given the current disadvantages with guided tissue regeneration, we saw the need to develop a new class of membranes, which have tissue and bone regeneration properties along with a flexible coating that can adhere to a range of biological surfaces,” said Alireza Moshaverinia, DDS, MS, PhD, lead author of the study and assistant professor of prosthodontics at the UCLA School of Dentistry.
“We’ve also figured out a way to prolong the drug delivery timeline, which is key for effective wound healing,” Moshaverinia said.
The researchers started with a polymer approved by the Food and Drug Administration, a large-scale synthetic molecule commonly used in biomedical applications. Its surface isn’t suitable for cell adhesion in periodontal treatment, so the researchers introduced a polydopamine coating.
A polymer with excellent adhesive properties, the coating can attach to surfaces in wet conditions. It also speeds up bone regeneration by promoting the mineralization of hydroxyapatite, which is the mineral that makes up tooth enamel and bone.
After identifying an optimal combination for the new membrane, the researchers used electrospinning to bond the polymer with the polydopamine coating. Electrospinning simultaneously spins two substances rapidly with positive and negative charges and fuses them to create one substance.
To improve the new membrane’s surface a structural characteristics, the researchers used metal mesh templates in conjunction with the electrospinning to create different patterns, or micro-patterning, similar to the surface of gauze or a waffle.
“By creating a micropattern on the surface of the membrane, we are now able to localize cell adhesion and to manipulate the membrane’s structure,” said co-lead author Paul Weiss, PhD, presidential chair and distinguished professor of chemistry and biochemistry, bioengineering, and materials science and engineering.
“We were able to mimic the complex structure of periodontal tissue and, when placed, our membrane complements the correct biological function on each side,” said Weiss.
To test the membrane’s safety and efficiency, the researchers injected rat models with gingival-derived human stem cells and human periodontal ligament stem cells. Next, they spent eight weeks evaluating the degradation of the membranes and the tissue’s response.
After the eight weeks, the patterned, polydopamine-coated polymer membrane had higher levels of bone gain when compared to models with no membrane or a membrane with no coating.
To suit a wide range of medical and dental applications, the researchers also discovered that adding and subtracting different oxidative agents or using lighter polymer bases before going through the electrospinning process can adjust the speed at which their membranes degraded when inserted in their models. This ability to turn degradation rates up or down helped the researchers control the timing of the delivery of drugs to the desired areas.
“We’ve determined that our membranes were able to slow down periodontal infection, promote bone and tissue regeneration, and stay in place long enough to prolong the delivery of useful drugs,” said Moshaverinia.
“We see this application expanding beyond periodontitis treatment to other areas needing expedited wound healing and prolonged drug delivery therapeutics,” said Moshaverinia.
Next, the researchers will evaluate whether their membranes can deliver cells with growth factors in the presence or absence of stem cells. The research was supported by a grant from the National Institute of Dental and Craniofacial Research. The study was published by ACS Nano.
Related Articles
Single-Unit Cement-Retained Implant Restorations
Protein Combination Improves Bone Regeneration
Nanodiamonds Improve Root Canal Therapy


