Industry News
Exocad Releases Exoplan 3.0 Galway Software in the United States
exocad, an Align Technology, Inc. company, and a leading dental CAD/CAM software provider, has...
Industry News
Navident 3 Receives FDA Clearance
ClaroNav Inc. announced that it has received 510(k) clearance from the US Food and Drug Administration (FDA) for the...
AI in DentistryTodays Dental News
VideaHealth Dental AI Solution Receives FDA 510(k) Clearance
VideaHealth, a dental AI pioneer, has announced the U.S. Food and Drug Administration (FDA)...
AI in DentistryTodays Dental News
FDA Clears World’s First AI Software to Read Dental X-Rays
Pearl, the leader in dental AI solutions, has announced that the United States Food...
AI in Dentistry
SoftSmile Announces FDA 510(k) Clearance for AI Powered Software VISION
SoftSmile, a leading medical technology company based in NY, and developer of advanced orthodontic...
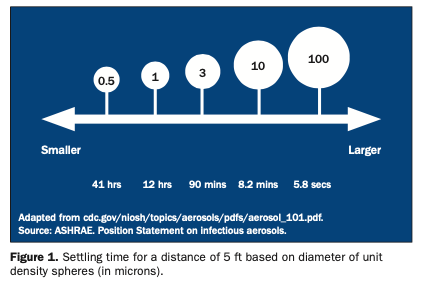
Infection Control
Controlling Pathogens in Your Air
During the 20th century, significant advances were made worldwide in health care and the...
Industry News
SprintRay to Celebrate International Dental 3D Printing Day on December 3, 2021
SprintRay Inc., an industry leader in digital dentistry and 3D printing solutions, has announced...
Industry News
SprintRay Receives FDA 510(k) Clearance for NightGuard Flex
SprintRay Inc., an industry leader in digital dentistry and 3D printing solutions, has announced...
Industry News
Mercury Safety Course For Dentists Launched In Multiple Languages
The International Academy of Oral Medicine and Toxicology (IAOMT) is pleased to announce that its...
Industry News
FDA Grants 510(k) Market Clearance to Vivos Therapeutics’ mmRNA Oral Appliance for Treating Mild to Moderate Sleep Apnea
Vivos Therapeutics, Inc. (the “Company” or “Vivos”) (NASDAQ: VVOS), a medical technology company focused...