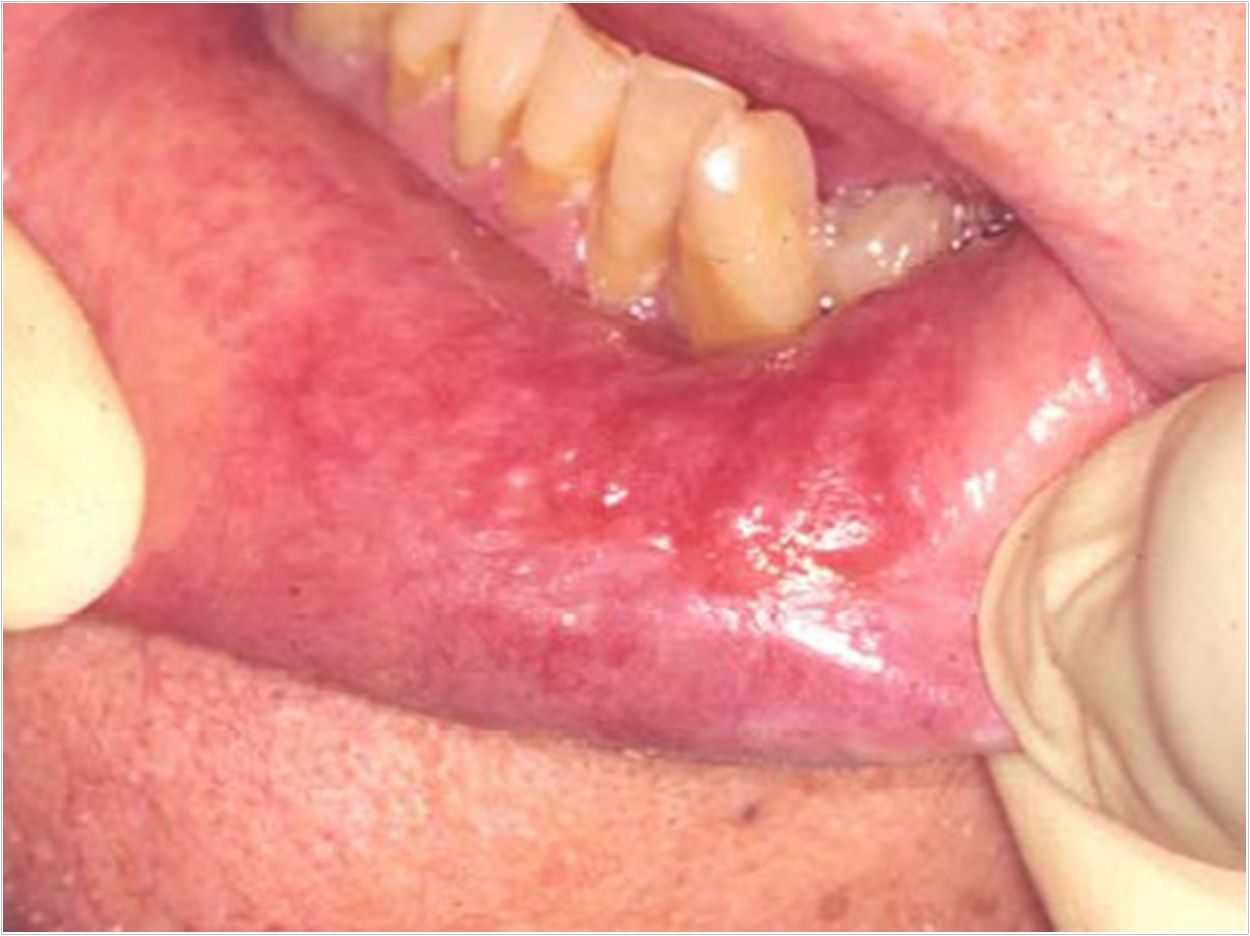
The Rivelin Clobetasol patch (Rivelin-CLO) from AFYX Therapeutics has met the primary and secondary endpoints in a Phase 2b study in patients with oral lichen planus (OLP). In the largest randomized, double-blind, placebo-controlled study ever conducted in OLP patients, the company said, the 20-µg dose (Phase 3 recommended dose) demonstrated a statistically significant improvement in the ulcer area and continued to show improvement through the end of four weeks.
Secondary endpoints focused on capturing patient symptomatic improvement also demonstrated statistical significance, AFYX Therapeutics said, further validating the clinical benefit observed in these patients. No serious adverse events were observed, and patients reported overall ease of use and patch adherence to the oral cavity for an average of 90 minutes.
“We are excited by the results of this Phase 2b study of Rivelin-CLO, which clearly demonstrated first-of-its-kind therapeutic benefits with OLP, a condition for which no approved therapies exist,” said Nishan de Silva, MD, CEO of AFYX Therapeutics.
“Investigators we have been working with have expressed challenges with existing treatment options—topical treatments, ointments, and mouth rinses that don’t provide adequate results—and view Rivelin-CLO as an important potential solution for addressing these unmet needs. Based on the strength of these results, we plan to move 20-µg Rivelin-CLO into Phase 3 clinical studies. We look forward to sharing more detailed results at a future medical/scientific meeting,” said de Silva.
Rivelin-CLO is the first biodegradable oral adhesive patch designed for local delivery of clobetasol to treat symptomatic OLP lesions. It uses a novel electrospinning technology to create a unique patch capable of adhering to the oral cavity for an average of 90 minutes and other wet tissue surfaces for approximately 9.5 hours while delivering a steady therapeutic dose to the lesion, the company said.
OLP is a chronic, inflammatory disease characterized by symptomatic lesions and ulcers in the mouth. It is estimated to affect more than 1% of the population in the United States and Europe, or over 6 million patients combined, AFYX Therapeutics said. There are no approved treatments for OLP.
“Rivelin-CLO adhered to wet tissue inside the mouth of patients with OLP, delivered a steady dose of clobetasol that led to a significant reduction in wound size, and patients were able to easily adhere to the treatment regimen,” said principal investigator Michael Brennan, DDS, MHS, professor and chair of the Department of Oral Medicine at Atrium Health’s Carolinas Medical Center in Charlotte, North Carolina.
“This is very encouraging data for a disease that has been overlooked, and I look forward to further evaluating Rivelin in Phase 3 studies,” said Brennan.
The Phase 2b trial enrolled 138 adult patients diagnosed with OLP and who had at least one visible and measurable symptomatic ulcerative lesion. Conducted in Europe and North America, the study used three active dose arms (Rivelin-CLO 1-µg patch, 5-µg patch, and 20-µg patch) and one placebo arm. Patients were treated twice daily and evaluated on a weekly basis for 28 days of treatment.
Rivelin is a muco-adhesive, two-layered patch that delivers a pharmaceutical product such as clobetasol directly to wet tissue surfaces. Its unique patch technology adheres to mucosal surfaces for extended periods, the company said, facilitating unidirectional delivery of a pharmaceutical agent to the target site of action impacting disease progression while limiting delivery to surrounding areas. This should enable higher efficacy, lower dosing, and less toxicity for nonaffected parts, AFYX Therapeutics said.
Rivelin also may significantly improve the treatment paradigm for OLP and other inflammatory mucosal diseases, the company added, which today are largely addressed with unapproved or inconvenient ointments, inhalers, or mouthwashes.
Related Articles
Recognize the Patient and Lesion Risk Factors for Oral Cancer
Adhesive Patch Treats Oral Lesions More Effectively Than Creams
Bioadhesive Patch Chases Canker Sores Away












