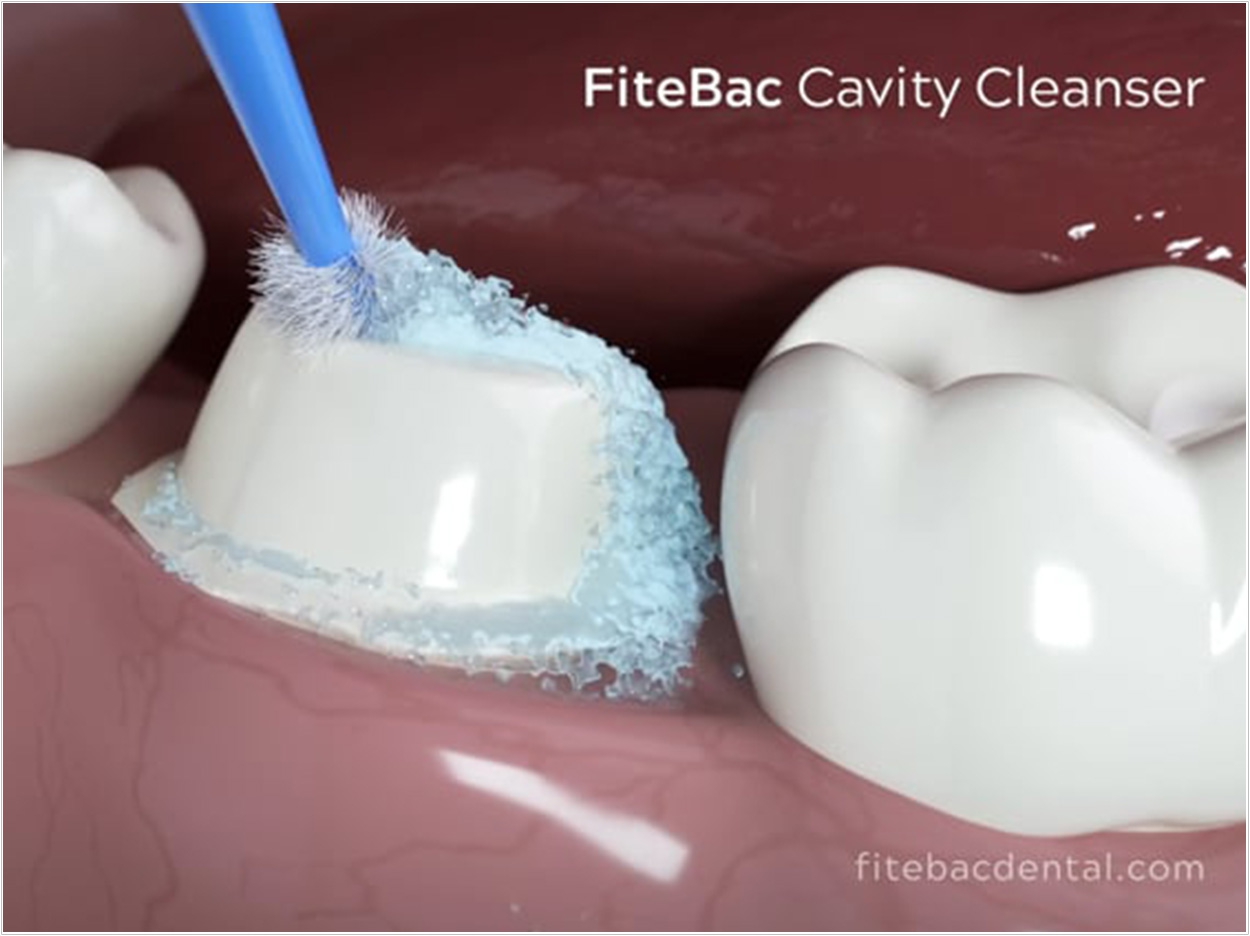
The Food and Drug Administration (FDA) has cleared Largent Health’s FiteBac Antimicrobial Cavity Cleanser with 2% K21 for marketing. Dental cavity cleansers are used for cleansing and moistinenting/rewetting prepared dental surfaces prior to completion of tooth restoration.
FiteBac technology exemplifies the marriage of antimicrobial technology to material science, the company said, enabling the manufacture of modern materials offering sustained, non-leaching antimicrobial protection across a broad range of products.
FiteBac Antimicrobial Cavity Cleanser with 2% K21 is intended to become part of the restoration and dentin interface by copolymerizing with the dental adhesive. It helps remove debris in carious lesion preparation and reduce the presence of dentally relevant organisms within the prepared tooth structure and to penetrate exposed dentin tubules, allowing restorative adhesives to tightly bind to the prepared dentin surface, the company said.
“With the introduction of FiteBac Antimicrobial Cavity Cleanser, we believe we can offer dentists a differentiated product to help the dental professional ensure the restoration site is clear of carious debris as well as to assist in directly addressing residual microbial contamination within the prepared tooth structure,” said Jude Dinges, chief commercial officer at Largent Health.
Related Articles
Correction of Extremely Dark Anterior Teeth


