 |
|
Photo: Getty Images
|
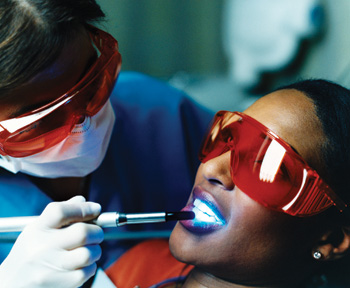
| Occupational Safety and Health Association requires provider and patient to wear proper eyewear to protect against airborne infections |
The most recent comprehensive infection control guidelines and recommendations for dentistry were published in 2003.1 An “evidence-based” format was utilized in the development of this document, including inclusion of initial sections which reviewed published epidemiological, clinical, and scientific information related to the specific recommendations presented later in the publication. Even though the 2003 US Center for Disease Control (CDC) Guidelines represent continued application of updated practices, procedures, and products; the essential component for success of an infection control program remains compliance. Published surveys have suggested that, although adherence to documented evidence-based recommendations is effective in limiting the potential for accidental occupational exposures, compliance with published guidelines and regulations can be an issue.2-4 Questions by conscientious healthcare providers continue to be asked concerning how to accomplish practical applications of published recommendations, sometimes with the mistaken perception that there is only one single approach to achieving infection prevention goals. In addition, use of practices that are unsupported by accumulated scientific evidence, or inferential application of those data, can also reduce compliance and lead to later problems. Instead, what we need to remember is that while effective infection control requires application of basic principles, healthcare professionals should realize that there are reasonable choices in a number of areas when they develop practice protocols and when choosing available products.
Question 1. I was taught in school to use a “cold sterilization” solution for reprocessing certain instruments. I see that this practice is not recommended in recent infection control guidelines. What is different today from years ago?
In healthcare settings, cold sterilization refers to the practice of immersion (liquid chemical) disinfection used to reprocess reusable instruments or items for patient care. This practice was not a true sterilization procedure which could be biologically monitored and is no longer appropriate for reprocessing heat-stable medical instruments. In addition, “chemical immersion sterilants” such as glutaraldehydes can be toxic and allergenic to those healthcare workers who use them. Dentistry has very little (if any) use for these types of chemical agents. As a result of improved technology and manufacturing, virtually every reusable dental instrument in current use is heat-stable, and thus, should be appropriately cleaned, wrapped, and sterilized between uses with a heat-based, biologically monitored sterilizer, such as an autoclave, unsaturated chemical vapor sterilizer, or dry heat sterilizer.
Question 2. After I use the alcohol-based hand antiseptic in my office, I notice that my hands feel slippery and do not feel clean. Why does that happen and what can I do to take remedy this?
Repeated use of high concentrations of alcohol found in available water-free hand sanitizers/antiseptics can have a drying effect on the skin. People with sensitive skin can be especially affected. For this reason, products tested and approved for routine use in health professional facilities contain emollients, such as glycerin or aloe vera. These important additives reduce the drying potential of the antimicrobial alcohol over time with repeated use. Some personnel may feel a “buildup” of these emollients after a few uses of the hand sanitizer since water is not involved in this hand hygiene process. If the presence of the film bothers them, they merely need to wash their hands with soap and water to remove it. There isn’t any hard and fast regulation about how often to wash the emollients off. It is up to the individual to determine the frequency, but keep in mind that they do help to keep the keratinized epithelium on hands intact.
Question 3. I received the hepatitis B vaccine in the late 1980s. I was tested to be sure that I had developed antibodies soon thereafter, and was found to be positive. I go for a blood check every few years and recently the results came back negative for antibodies. Do I need a booster?
As of this writing there is no recommendation for a hepatitis B vaccine booster dose. The important thing is that you were tested after receiving the 3-dose regimen, and demonstrated a positive protective serologic titer for anti-hepatitis B surface antibodies (anti-HBs). This titer can decline over a period of years when the person is not accidently exposed to the hepatitis B virus. Ongoing studies of persons who responded to the vaccine indicate that immunologic memory remains intact for at least 23 years conferring protection against clinical illness and chronic hepatitis B virus infection, even though anti-HBs levels might become low or decline below detectable levels. What is important here is that you did get tested for antibodies after receiving the vaccine series. That way you knew your immune system had responded. The fact that immunological memory is lasting more than 20 years (and still going) reinforces the long-term value of this important vaccine.
Question 4. I have a dental assistant who has been working in the profession for many years. She tells me that she really dislikes receiving injections. She does not want to receive the hepatitis B vaccine, even though I would pay for it. In her mind she assumes that she was exposed to “everything” years ago and does not need the vaccine. Can she refuse vaccination even though I am required to offer and provide it to her?
She does have the right to refuse the vaccination, but she should know that without the vaccination series or serologic evidence of prior infection, she remains at risk for occupational viral infection. Federal and State Occupational Safety and Health Association (OSHA) regulations consider hepatitis B vaccination one of the most important protective measures a health professional can have, and she is required to sign an informed refusal waiver if she does not want to receive it. However, please remember that if she changes her mind in the future, and is still working as your employee, you are required to pay for the vaccine.
Question 5. Do prescription glasses provide sufficient eye protection when treating patients?
The trend in personal eyewear has rapidly shifted in recent years to the use of smaller, designer-oriented frames. Many of these do not adequately cover the eye
s to protect against the routine macroscopic and microscopic exposures that occur during dental patient treatment. Thus, while they may be fashionable, these glasses are not sufficient for protection of the healthcare worker. Health professionals were very compliant with using appropriate eyewear shortly after the initial federal OSHA regulations were published in December 1991. Over the years, however, as less and less ocular injuries and infections have been reported from occupational exposures, personnel have gradually forgotten about the serious ramifications that can occur after airborne exposure to tooth particles, restorative materials, and salivary droplets. Everyone should be reminded that the eyes are very susceptible to injury and that they need to be covered completely with eyewear that is designed to be large enough and includes side shields. If one still wants to wear personal glasses or contacts, disposable eyewear is available which is both easy to use and provides protection during patient care. The important message to take away from this is the necessity to protect the least protected part of the body during dental care—your eyes.
Question 6. Does the vaccine against 2009 to 2010 seasonal influenza also provide cross-protection against the A/H1N1 “swine” pandemic flu virus?
No, cross-protection is not provided between the 2009 and 2010 seasonal influenza and A/H1N1 pandemic vaccine preparations. The pandemic “swine flu” virus is very different genetically from seasonal types. The latter constantly change by a process called “antigenic drift.” This alteration results from minor point mutations in the viral genetic material during replication in host cells. As a consequence, these viruses can routinely change from one season to the next, or in some instances, even within a single flu season. In contrast, pandemic influenza strains arise from a different mechanism involving genetic recombination between multiple influenza A virus subtypes. This process, termed “antigenic shift,” occurs from influenza viruses from different species co-infecting the same animal host (ie, the pig), whose cells have receptors for human, bird, and swine flu viruses. Whole segments of viral nucleic acid can be exchanged in creating progeny viruses. The resultant viruses contain segments of RNA from each of the original viruses, are typically are completely different in their antigenic makeup from any previously known strain, and often are more virulent than seasonal flu viruses. When new influenza A viruses are introduced into the human population, infected persons have little or no protective immunity from previous exposure to other influenza viruses. In the worst case scenario, when these strains adapt to efficient human-to-human transmission, the potential for a widespread pandemic arises with a high mortality rate.
Question 7. Can injected seasonal influenza vaccines give you the flu?
No, the vaccine does not cause all people to develop influenza after immunization. Unfortunately, this is a common misconception concerning the efficacy and safety of flu vaccines. The influenza viruses used in vaccine preparations are grown in chick embryo tissue cultures and then harvested. These viruses are inactivated with formalin. These “killed” viruses are then split into components to yield the final preparation. Batches of vaccine are tested to ensure safety before they are approved for human immunization. According to the CDC, in randomized, blind investigations, where some participants received flu shots while others were injected with saline, the only difference in symptoms noted was an increase in soreness in the arm and injection site soreness among those who received the flu shot. No differences were noted between the 2 groups with regard to body aches, fever, cough, or sore throat.
Question 8. I have been receiving information about a new autoclave technology called Class B sterilizers. How do they work? Are they more efficient than the autoclaves currently in use?
This European-developed innovation in sterilizer technology arrived in the United States about 10 years ago and is suitable for all dental practices. While Class B sterilizers (also called prevacuum and postvacuum steam sterilizers) are similar to gravity displacement autoclaves (they use steam under pressure to sterilize contaminated items), they are also fitted with a pump that creates a vacuum in the chamber to ensure air is removed from the sterilizing chamber before steam enters. The advantage here is that very rapid steam penetration occurs throughout the chamber because the steam does not mix with air before reaching packaged instruments. As a result, there is faster and more thorough steam penetration throughout the entire load, with the result being a shortening of the required time for instrument sterilization. Class B sterilization cycles also can minimize a common problem seen in many practices: this is the issue of finding wet instrument packs at the end of a sterilization cycle which occurs when dental personnel overload the chamber prior to beginning the cycle. The pre and postvacuum autoclaves address this by having a poststerilization vacuum cycle that facilitates drying by removing steam at the end of the sterilization portion of the cycle. This important feature provides dry instrument packs at the end of the process. Although larger versions of these pre and postvacuum sterilizers have been available for a number of years in hospitals, dental schools, and large clinics, smaller tabletop units have become available in the United States in recent years and can be very useful as components of an efficient instrument reprocessing protocol.
Question 9. There are numerous barriers, sprays, and wipes available for accomplishing effective environmental surface infection control. What are some of the mistakes that can occur in this area, thereby compromising the effectiveness of the procedures used to accomplish surface asepsis?
Representative problems can include:
a. Not cleaning contaminated surfaces prior to disinfection. The importance of initial cleaning cannot be overemphasized, as it is included routinely in all published infection control recommendations. Cleaning is the physical removal of debris which also results in reduction of the number of microorganisms present on the inanimate surface.
b. Reuse of barriers sold as single-use items.
c. Use of inappropriate products as surface disinfectants. Some individuals previously proposed the use of glutaraldehydes (ie, high-level disinfectants) on contaminated environmental surfaces. This would constitute a potentially harmful misuse of this immersion type of chemical. These solutions are not manufactured to be used as surface disinfectants and can pose significant health risks for healthcare personnel who spray them onto surfaces.
d. Mistakenly substituting products that have not been tested and approved for use in healthcare facilities. Household cleaners and sanitizers do not undergo the same rigorous testing, quality control, and Environmental Protection Agency (EPA) approval process as those that are approved for use in dental and medical settings. Manufacturers of surface disinfectant sprays and disposable wipes must conduct a number of required independent laboratory, toxicity, and compatibility studies as part of the EPA evaluation process.
e. Over spraying of disinfectants. Misuse/overuse of disinfectants can occur in several ways. Operatory surfaces that are repeatedly overexposed to chemicals can discolor, or equipment can be damaged. An even more serious problem can arise in addition to equipment damage, whereby personnel can develop respiratory symptoms, such as sneezing or wheezing when the chemical disinfectant is sprayed. Ocular irritation, headaches, and even allergies to the chemical can also occur from excessive spraying.
T
he bottom line here is to be certain to read and follow the manufacturer’s product label. The content of the label also needs to be approved by the EPA before the product receives an EPA number.
CLOSING COMMENTS
In summary, I am reminded of a question that was posed to me recently. The question was: What advice can dental professionals use concerning the implementation and maintenance of a practical infection control program? The bottom line for many aspects of an effective program is to use common sense. You can readily get confused with some of the rhetoric, available products, and “improved” technologies. It is important to focus on what you are trying to accomplish.
References
- Kohn WG, Collins AS, Cleveland JL, et al, and the Centers for Disease Control and Prevention (CDC). Guidelines for infection control in dental health-care settings—2003. MMWR Recomm Rep. 2003;52(RR-17):1-61.
- Siew C, Gruninger SE, Miaw CL, et al. Percutaneous injuries in practicing dentists. A prospective study using a 20-day diary. J Am Dent Assoc. 1995;126:1227-1234.
- McCarthy GM, Koval JJ, MacDonald JK, et al. Compliance with recommended infection control procedures among Canadian dentists: results of a national survey. Am J Infect Control. 1999;27:377-384.
- Bischoff WE, Reynolds TM, Sessler CN, et al. Handwashing compliance by health care workers: The impact of introducing an accessible, alcohol-based hand antiseptic. Arch Intern Med. 2000;160:1017-1021.
Dr. Molinari is currently Director of Infection Control for THE DENTAL ADVISOR in Ann Arbor, Mich. Previously, he was a full-time faculty member at the University of Detroit Mercy School of Dentistry for 32 years, where he served as Professor and Chair of the Department of Biomedical Sciences. He has published more than 350 scientific articles, text chapters, and abstracts in the areas of microbiology and immunology, and lectures nationally and internationally on topics dealing with infectious diseases and infection control. Dr. Molinari is also co-author of the text Cottone’s Practical Infection Control in Dentistry, with the third edition published in 2009. His activities also include serving as a consultant for the CDC, ADA Council on Scientific Affairs, Council on Dental Practice, and hospitals in the Detroit area in the areas of infectious disease and infection control Dental Association. He can be reached at johnmolinariphd@gmail.com.
Disclosure: Dr. Molinari is a consultant for Hu-Friedy Manufacturing and SciCan.



