Synthetic biologists at Northwestern University have developed a simple and inexpensive test that can detect dangerous levels of fluoride in drinking water, compared to current tests that cost hundreds of dollars and often require scientific expertise to use.
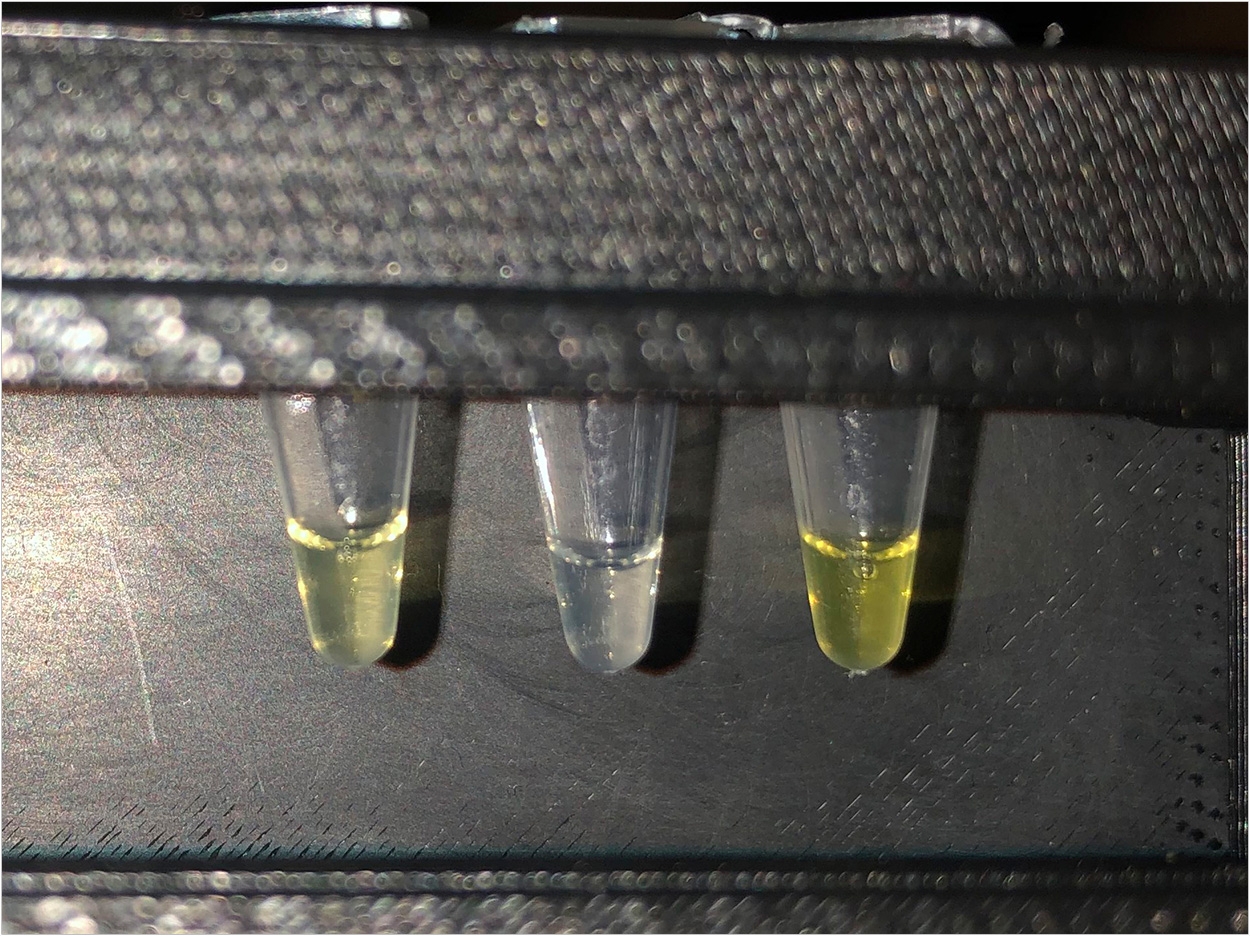
Costing just pennies to make, the system only needs a drip and a flick, the researchers said. Users drip a tiny drop of water into a prepared test tube, flick the tube once to mix it, and wait. If the water turns yellow, then an excessive amount of fluoride exceeding the Environmental Protection Agency’s most stringent regulatory standards is present.
The researchers tested the system both in the laboratory at Northwestern and in the field in Costa Rica, where fluoride is naturally abundant near the Irazu volcano. When consumed in high amounts over long periods of time, fluoride can cause skeletal fluorosis, a painful condition that hardens bones and joints.
Americans tend to think of the health benefits of small doses of fluoride that strengthen teeth, the researchers said. But elsewhere in the world, specifically across parts of Africa, Asia, and Central America, fluoride naturally occurs at levels that are dangerous to consume.
“In the United States, we hear about fluoride all the time because it’s in toothpaste and the municipal water supply,” said project leader Julius Lucks, PhD, MS, MPhil, associate professor and chair of chemical and biological engineering.
“It makes calcium fluoride, which is very hard, so it strengthens our tooth enamel. But above a certain level, fluoride also hardens joints. This mostly isn’t an issue in the US. But it can be a debilitating problem in other countries if not identified and addressed,” said Lucks.
Fluoride is a naturally occurring element that can seep out of bedrock and into groundwater. It also is found in volcanic ash and is particularly abundant in regions surrounding volcanoes. Home to three volcanic range systems, Costa Rica seemed like a natural fit to test the device.
Matthew Verosloff, a PhD candidate in Lucks’ laboratory, traveled to Costa Rica and sampled various sources of water, including mud puddles, ponds, and ditches.
“Every test on these field samples worked,” Lucks said. “It’s exciting that it works in the lab, but it’s much more important to know that it works in the field. We want it to be an easy, practical solution for people who have the greatest need. Our goal is to empower individuals to monitor the presence of fluoride in their own water.”
Although the device is simple to use, the prepared test tube houses a sophisticated synthetic biology reaction. Lucks has spent years working to understand RNA folding mechanisms. In his new test, he puts this folding mechanism to work.
“RNA folds into a little pocket and waits for a fluoride ion,” said Lucks. “The ion can fit perfectly into that pocket. If the ion shows up, then RNA expresses a gene that turns the water yellow. If the ion doesn’t show up, then RNA changes shape and stops the process. It’s literally a switch.”
According to Lucks, organisms already perform this function in nature.
“Fluoride is toxic to bacteria,” he said. “They use RNA to sense fluoride in the cell. Then they make a protein to pump it out and detoxify.”
Lucks’ system works in the same way. But instead of producing a protein pump, his test produces a protein enzyme that makes a yellow pigment, so people can see the results with a simple glance.
Lucks’ team freeze-dried the RNA reaction, which looks like a tiny cotton ball, and put it into a test tube. In this form, the reaction is safe and shelf-stable. A small pipette accompanies the test tube.
When placed in water, the pipette absorbs exactly 20 microliters, which is just the drop needed to rehydrate the reaction. From there, it takes two hours to get a result, which Lucks intends to accelerate in future iterations.
“We’re currently limited to testing for fluoride,” said Walter Thavarajah, a graduate student and the paper’s first author. “But we’re trying to engineer other RNAs to detect all sorts of targets.”
The study, “Point-of-Use Detection of Environmental Fluoride Via a Cell-Free Riboswitch-Based Biosensor,” was published by ACS Synthetic Biology.
Related Articles
Study Questions Value of Fluoride Varnish
Dental Groups Defend Fluoride After Study Implicates It in Lower IQs
Fluoride Linked With Diminished Kidney and Liver Function in Adolescents


