It has been estimated that as many as 60 million consumers in the United States have periodontal disease, an estimated 35% of the adult population needing care.1 It is now possible, utilizing evidence-based technology and supported care, that active periodontal disease with 6 mm of pocket depth or less can be controlled successfully and periodontal health maintained. Pocket depths greater than 6 mm are found in less than 5% of the population.1
It can be anticipated that more consumers, after learning they can be treated and stabilized without surgical intervention, will come to the dental office for this critically needed care. The first procedure that should be done for these new patients is a periodontal exam, to include a complete pocket depth probing.
The Oral-Systemic Connection
There is increasing evidence, with clinical studies having been completed and now underway, that periodontal disease may pose a significant risk to a patient’s health. The oral-systemic disease connection reached prominence with the report of the US Surgeon General, Dr. David Satcher, in May 2000. Donna Shalala, then Secretary of the US Department of Health and Human Services, remarked in the opening message that the terms oral health and general health should not be interpreted as separate entities, and that oral health is integral to general health. She stated that oral health means more than healthy teeth, and you cannot be healthy without oral health.2
Most consumers want to be healthy and do not want to risk their health by neglect. If there is an opportunity to reduce risk of systemic disease by improving dental health, they are likely to consider treatment once a diagnosis is made and the potential risks for nontreatment are identified to them. Once periodontal health is established following an evidence-based standard of care, restorative and cosmetic dentistry can be considered. The patient can have more confidence in this treatment, since successful periodontal treatment has been provided and a stable condition of the periodontal tissues achieved.
 |
 |
|
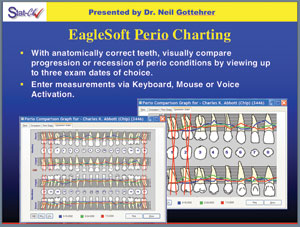
Figure 1. EagleSoft perio charting, with areas requiring treatment highlighted in red. |
Figure 2. A colored probe makes it easy for the patient to see probing markings. |
Periodontal therapy outcomes can be improved through several methods. Bennett3 recently reviewed the use of power scalers to improve periodontal outcomes. She concluded that a new understanding of the cause and progression of periodontal disease has led to dramatic changes in treatment, recognizing that Gram-negative periodontal pathogens in oral biofilms are necessary to initiate and continue disease progression. Thomas4 describes the dental plaque biofilms as the microbial “Trojan horse” that attacks tissue as an adhesive mass with significant inflammatory disease potential that cannot be eliminated. Disruption and control of these biofilms are necessary to initiate and disrupt disease progression, with this disruption being essential to establishing health.
With the knowledge of the multiple components of periodontal disease progression, Gottehrer and Shirdan5 introduced in 2002 the StatCk System, a new guide for nonsurgical management of periodontal disease. It includes an abbreviated probing system to document the patient’s condition and to aid in diagnosis and planning appropriate treatment, making treatment easier and more efficient. It allows the dentist/hygienist to predictably manage the patient with 4 to 6 mm of pocketing. This newer system for nonsurgical antimicrobial host response is now available on the Patterson Dental EagleSoft Verson 13.006 (Figure 1). With this type of data collection system, accurate recordings of periodontal health assessment parameters can be made. This offers graphic data printouts that can provide educational demonstrations for the patients who need treatment. The newer Colorvue Probe (Hu-Friedy) can be used (Figure 2), which shows the patient the probe markings visually in the mouth as they are recorded in the data collection system. With our ability to collect diagnostic data and assimilate clinical findings, we can now use this information to improve our care for patients and potentially reduce dental risk for cardiac disease.
The connection between periodontal disease and cardiac disease is now clearly established. Beck, et al7 stated in 2005 that “we reviewed evidence indicating that the chronic inflammatory burden of periodontal infection and the host response provide the basis for the observed association between periodontal disease and atherosclerosis and coronary heart disease (CHD). Thus we conservatively interpret the results of this study to indicate that systemic exposure to oral organisms is related to the prevalence of detected CHD.”
Desvarieux, et al8 in 2005 also reported an association between periodontal disease and cardiovascular disease. They studied the relationship between the carotid artery intimamedia thickness (IMT) and periodontal microbiota. They found a positive independent relationship between carotid IMT and cumulative periodontal bacterial burden. They showed that the observed relationship with the carotid IMT reflects both the burden and dominance of those pathogens etiologically related to periodontal disease in the subgingival microbial niche. They concluded that their findings strengthen the hypothesis that oral infections may contribute to cardiovascular disease morbidity and bolster the supposition that accelerated atherosclerosclerotic development is a possible mechanism connecting chronic infections and cardiovascular disease.
Similarly, there appears to be a direct connection of periodontal disease to diabetes. Since periodontal disease is inflammatory, it may alter glycemic control. Taylor, et al9 showed that diabetic patients with periodontal infection have a greater risk of worsening glycemic control over time compared to diabetic subjects without periodontitis.
Saremi, et al10 recently completed a longitudinal study examining the effect of periodontal disease on mortality from multiple causes in more than 600 patients with type 2 diabetes. The death rate from ischemic heart disease was 2.3 times higher in patients with severe periodontal disease than the rate in patients with no periodontitis or only slight disease, after accounting for other known risk factors. The death rate from diabetic nephropathy was almost 8.5 times higher in patients with significant periodontal disease, and the overall mortality rate from cardiac renal disease was 3.5 times higher in patients with severe periodontitis.
The World Health Organization11 has stated that cardiovascular disease accounts for 29% of deaths worldwide and ranks as the second leading cause of death after infections and parasitic diseases. The American Heart Association has reported that atherosclerosis, a major component of cardiovascular disease, affects 1 in 4 persons and contributes to 39% of deaths annually in the United States.12
The traditional major risk factors now recognized for cardiac disease are cigarette smoking, hypertension, high levels of low-density lipoprotein cholesterol, low levels of high-density lipoprotein cholesterol, diabetes mellitus, family history of premature coronary heart disease, age (men <45 years, women <55 years), obesity, physical inactivity, and an atherogenic diet.13 Knowing of the association between periodontal disease and cardiovascular disease, and the notable incidence of periodontal disease in the cardiovascular disease population, these risk factors may soon be officially joined by periodontal disease.
REDUCING THE DENTAL RISK FOR CERTAIN SYSTEMIC DISEASES
 |
|
Figure 3. Type F Stat-Ck is a grade F patient. |
The standard of care allows for an anticipated predictable and successful long-term stabilization of the patient’s periodontal condition. A standard of care for nonsurgical management of periodontal disease to reduce the dental risks for certain systemic diseases (noted above) is required if we, as professionals, are to produce highly predictable results in periodontal management, due to the severity of the health factors involved. A typical advanced condition (Figure 3), classified grade F in the Stat-Ck system previously described, can be successfully managed using this standard of care.
This suggests that site-specific treatment be utilized to manage the disease, supported by evidence-based technology. This includes the use of the following:
- antimicrobial toothpaste
- antimicrobial mouthwash
- hygiene devices including power brushes
- power irrigation
- ultrasonic and hand instrumentation
- placement of time-released, locally applied antimicrobial drugs below the gingival margin into periodontal pockets with 5 mm of depth or more and bleeding on probing
- site management of the medically compromised patient to control bleeding6
In addition to this, routine use of inflammatory modulators such as low-dose doxycycline 20 mg (not acting as an antibiotic) to address local tissue inflammation via the systemic route is required for patients with adult periodontitis, to improve the efficacy of scaling and root planing using ultrasonics and hand instrumentation, as shown by Caton, et al in 2000.14
Ridker documented that the degree of inflammation, as measured by high-sensitivity C-reactive protein (CRP), correlates with the prognosis of patients with established coronary disease.15 A benefit of using low-dosage doxycycline via the systemic route for patients with coronary artery disease also has been demonstrated. Brown16 conducted a clinical study (double-blind, placebo controlled) of 50 patients with coronary artery disease, administering subantimicrobial doses of doxycycline (SDD/20 mg twice daily). CRP was reduced 46% in the study group, and not significantly reduced in the control group. No periodontal treatment was provided.
While use of interproximal cleaning with dental floss is still encouraged, recent evidence shows that use of a powered toothbrush appears to be as effective for most adults as manual toothbrushing combined with interproximal cleaning.17 As a result of this, patients should be encouraged to use a power toothbrush as part of their daily oral hygiene routine.
Antibacterial toothpastes (eg, Colgate Total [Colgate-Palmolive]) have been documented to significantly reduce microorganisms from the tongue, plaque, and saliva, and produce sustained effects on these oral bacteria for 12 hours.18 These pastes should be used at least twice a day with brushing, and patients should be encouraged to routinely use them.
Cobb19 demonstrated that irrigation cleansed deep between the teeth and below the gingival margin to remove bacteria associated with gum disease. Since it has been shown that the pathogenic nature of dental plaque can be reduced by reducing the total microbial load, maintaining a normal flora using an antimicrobial mouthwash (eg, Listerine [McNeil-PPC]) can result in prevention of periodontal disease.4 As with the antimicrobial toothpaste, the antimicrobial mouthwash, preferably delivered below the gingival margin using the power irrigator to reach the subgingival bacteria (completing the removal of the dental plaque), should be done twice daily.
The Institute for Advanced Oral/Physical Health, a nonprofit group located in suburban Philadelphia, is devoted to answering the questions about interplay of periodontal disease, the host immune and inflammatory responses, and the resulting clinical signs of this complex exposure that may affect general health. It is in the process of conducting an outcome assessment study, “Cumulative Periodontal Bacterial Burden: The Role it Plays in Cardiovascular Disease and Diabetic Control.” Lessening the cumulative periodontal burden on patients who have had cardiac stents placed will be evaluated for its effect on inflammatory biomarkers and patient mortality. The Institute will also act as a consumer advocate group for improved oral/physical health.
The Institute reviewed case histories of more than 1,000 patients treated using the above standard of care, which included use of an antimicrobial rinse in the power irrigator to gain subgingival action of the rinse to further reduce the bacteria present due to its antibacterial action. The clinical outcomes showed reduced areas of bleeding on probing and an overall improvement in clinical periodontal health (personal communication; Dr. Neil Gottehrer).
Cobb,20 in a comprehensive review of the literature in 2002, examined the reduction in probing depths and changes in attachment levels due to mechanical instrumentation with scaling and root planing. Pockets measuring 4 to 6 mm experienced a mean reduction probing depth of approximately 1.29 mm with a net gain in attachment of 0.55 mm. Periodontal pockets that had an initial probing depth >7 mm experienced reduction of mean probing depth of 2.16 mm and a gain in attachment of 1.19 mm.
Killoy21 in 2002 concluded that the local delivery of antimicrobials, eg, Arestin (minocycline HCl microspheres [OraPharma]) offers the clinician a statistically and clinically significant option in the treatment of chronic periodontitis. The Agency for Healthcare Research and Quality (AHRQ) is the federal agency assigned to improve the quality, safety, efficiency, and effectiveness of healthcare by evaluating literature through a systematic review process. AHRQ evaluated literature on antimicrobials in which SRP, accompanied by an antimicrobial agent as a supplemental or adjunct treatment, resulted in improved outcomes in adults with chronic periodontitis, as compared with SRP alone. The conclusion was that studies of locally applied antimicrobials, eg, minocycline, have fairly consistent positive results in moderately large studies.22 Thus, these antimicrobials should be used on a routine basis, as a standard of care, in managing periodontal disease.
In order to successfully implement a standard, all patients must be presented with a plan of treatment that will reduce and control the disease. The biggest obstacle to success in management of periodontal disease is not utilizing on a routine basis standards of care that will allow the professional to achieve the desired result. Once this is done, the professional can expect improved clinical results and a healthier patient.
One barrier to consumer acceptance of periodontal treatment is fear of pain. Treatment must be provided painlessly. Many times, in order to achieve the goal of painless treatment, analgesia must be used. While it has been estimated that 15% of the US population declines dental care primarily because they fear oral injections, nitrous oxide/oxygen analgesia relaxes patients and reduces their anxieties, allowing them to undergo treatment painlessly.6 The ADA recommends the use of a properly installed nitrous oxide delivery system with appropriate scavenging (eg, the Porter Instrument Conscious Sedation Flowmeter).23
 |
 |
|
Figure 4. Oraqix applicator tip is placed in gingival sulcus. |
Figure 5. BIDENT tip for coagulation. |
 |
|
Figure 6. BIDENT Bipolar Surgical System unit. |
To achieve a successful result with utilization of the standard of care, analgesia must be available to use with the patients who require it. A recent innovation in analgesia, making it possible to do nonsurgical treatment without injectable anesthesia, is Oraqix (DENTSPLY Pharmaceutical), a thermogel containing 2.5% lidocaine and 2.5% prilocaine. It is placed directly into the pocket using a blunt cannula (Figure 4) to place the anesthetic. It is not an injection, and its placement is relatively painless. It can take up to 30 seconds for onset and can last for an average of 20 minutes.
In providing periodontal treatment, following the guidelines of the standard of care, it is critical to be able to treat all patients with critical medical conditions on an immediate basis. Many of the patients who have suffered acute cardiovascular incidents are on anticoagulant drug therapy to reduce the risk of local clot formation. They are very susceptible to excessive bleeding even when receiving conservative nonsurgical periodontal care. Physicians are reluctant to remove these patients from the drugs they are taking to reduce the risk of clotting.
With the risk that some post-periodontal treatment involves bleeding for an extended time, the dental professional must be prepared to use a coagulating unit to prevent this bleeding (Figure 5). The post-treatment use of the medical BIDENT Bipolar Surgical System unit (Synergetics; Figure 6) permits the patient to remain on anticoagulants and prevents any excessive bleeding from occurring. This treatment must be done with the approval of the patient’s treating physician. It is an essential component of the standard of care if we are to be able to successfully manage all categories of patients.
Recognizing the significant medical risk of periodontal disease, it is imperative that every patient be evaluated for periodontal risk. Knowing that following the standard of care can allow the patient to be successfully managed for this disease, it is critical that the dental professional offer nonsurgical periodontal care to every patient who requires treatment. Know-ing the risk of periodontal disease and its association with serious medical conditions, most patients will consider and accept the necessary treatment.
If the prospective patient over age 50 with periodontal disease has bleeding on probing in pockets of at least 5 mm, it is now our obligation to this patient to suggest that he or she see a physician for a cardiac and diabetes risk assessment. The physician should be advised that the periodontal disease present in his or her patient is now considered a suspected risk factor for other systemic diseases, and that treatment is being provided because of the possible systemic risk factor. The results of any medical risk assessment should be requested and kept in the patient record.
This referral will convince the patient of our concern about their health and may also save a life!
References
- Albandar JM, Brunelle JA, Kingman A. Destructive periodontal disease in adults 30 years of age and older in United States, 1988-1994 [published correction appears in J Periodontol. Mar 1999;70:351]. J Periodontol. Jan 1999;70:13-29.
- US Department of Health and Human Services. Oral Health in America: A Report of the Surgeon General–Executive Summary. Rockville, MD: US Dept of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; 2000. http://www.nidr.nih.gov/AboutNIDCR/SurgeonGeneral/ExecutiveSummary.htm. Accessed October 10, 2007.
- Bennett BL. Using power scaling to improve periodontal therapy outcomes. Contemporary Oral Hygiene. 2007;7:14-21.
- Thomas JG, Nakaishi LA. Managing the Complexity of a Dynamic Biofilm. J Am Dent Assoc. 2006;137(suppl):10S-15S.
- Gottehrer NR, Shirdan TA. A new guide to nonsurgical management of periodontal disease. Dent Today. 2002;21:54-59.
- Gottehrer NR, Berglund SE. Antimicrobial host response therapy in periodontics. a modern way to manage disease, part 2. Dent Today. 2007;26:74-83.
- Beck JD, Eke P, Heiss G, et al. Periodontal disease and coronary heart disease: a reappraisal of the exposure. Circulation. 2005;112:19-24.
- Desvarieux M, Demmer RT, Rundek T, et al. Periodontal microbiota and carotid intima-Media Thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST). Circulation. 2005;111:576-582.
- Taylor GW, Burt BA, Becker MP, et al. Severe periodontitis and risk for poor glycemic control in patients with non-insulin-dependent diabetes mellitus. J Periodontol. 1996;67(suppl 10):1085-1093.
- Saremi A, Nelson RG, Tulloch-Reid M, et al. Periodontal disease and mortality in type 2 diabetes. Diabetes Care. 2005;28:27-32.
- World Health Organization. The World Health Report 1997: Con-quering Suffering, Enriching Hu-manity. Geneva, Switzerland: World Health Organization; 1997. http://www.who.int/whr/1997/en/index.html. Accessed October 11, 2007.
- American Heart Association. Heart Disease and Stroke Statistics – 2004 Update. Dallas, TX: American Heart Association; 2004.
- Smith SC. Current and future directions of cardiovascular risk prediction. Am J Cardiol. 2006;97(2A):28A-32A.
- Caton JG, Ciancio SG, Blieden TM, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol. 2000;71:531-532.
- Ridker PM, Hennekens CH, Buring JE, et al. Creactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836-843.
- Brown DL, Desai KK, Vakili BA, et al. Clinical and biochemical results of the metalloproteinase inhibition with subantimicrobial doses of doxycycline to prevent acute coronary syndromes (MIDAS) pilot trial. Arterioscler Thromb Vasc Biol. 2004;24:733-738.
- Axelsson P. Mechanical plaque control by self-care. In: Axelsson P. Preventive Materials, Methods, and Programs. Vol. 4. Karlstad, Sweden: Quintessence Publishing; 2004:80-101.
- Panagakos FS, Volpe AR, Petrone ME, et al. Advanced oral antibacterial/anti-inflammatory technology: a comprehensive review of the clinical benefits of a triclosan/copolymer/fluoride dentifrice. J Clin Dent. 2005;16(suppl):S1-S19.
- Cobb CM, Rodgers RL, Killoy WJ. Ultrastructural examination of human periodontal pockets following the use of an oral irrigation device in vivo. J Periodontol. 1988;59:155-163.
- Cobb CM. Clinical significance of non-surgical periodontal therapy: an evidence-based perspective of scaling and root planing. J Clin Periodontol. 2002;29(suppl 2):6-16.
- Killoy WJ. The clinical significance of local chemotherapies. J Clin Periodontol. 2002;29(suppl 2):22-29.
- Bonito AJ, Lohr KN, Lux L, et al. Effectiveness of Antimicrobial Adjuncts to Scaling and Root-Planing Therapy for Periodontitis. Summary, Evidence Report/Technology Assessment Number 88. Rockville, MD: Agency for Healthcare Research and Quality; 2004. AHRQ publication 04-EO14-1. http://www.ahrq.gov/clinic/epcsums/periosum.htm. Accessed October 11, 2007.
- Nitrous oxide in the dental office. ADA Council on Scientific Affairs; ADA Council on Dental Practice. J Am Dent Assoc. 1997;128:364-365.
Dr. Gottehrer has been in practice in suburban Philadelphia for more than 30 years, focusing his practice on cosmetics, implant dentistry, and periodontics. He is a graduate of the University of Maryland Dental School, received his postgraduate periodontal training at the University of Pennsylvania, and is a board-certified periodontologist. He teaches the Senior Elective Course in Periodontics at the University of Maryland Dental School. He has published and lectured nationally and internationally, and is currently the president of the Institute of Advanced Oral and Physical Health in Havertown, Pa. He can be reached at (610) 449-9500 or dr.neilg@verizon.net.
Dr. Martin has been in private practice in suburban Philadelphia for more than 25 years. He is currently Chief of Interventional Cardiology and Department of Cardiology, Main Line Hospitals, in Bryn Mawr, Pa. A graduate of Columbia University School of Medicine, having completed his cardiology fellowship training at the Hospital of the University of Pennsylvania, he is board certified in cardiovascular disease. He is Director of the cardiac catheterization lab, Bryn Mawr, Lankenau, and Paoli Hospitals of the Main Line Health System, having placed thousands of cardiac stents. He is currently associate professor of clinical medicine, Thomas Jefferson University, and is a Fellow of the American College of Cardiology, European Society of Cardiology, and the Society of Cardiac Angiography and Intervention. He has published and/or presented 75 papers on cardiovascular problems and is currently principal investigator in the Institute of Advanced Oral/Physical Health research study on the connection of cardiac and periodontal disease. He can be reached at (610) 331-5849 or MartinJ@MLHS.org.











