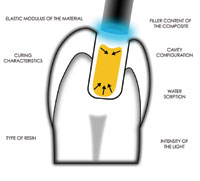
The use of hydrogen peroxide in dentistry can be traced back more than 100 years.1-4 Initially, hydrogen peroxide was evaluated for use in periodontal treatment and wound healing. Studies have substantiated that hydrogen peroxide can prevent and delay the colonization and replication of anaerobic bacteria.5,6 This reduction in the microflora, with subsequent reduction in the levels of plaque accumulation, resulted in better gingival health.7 Furthermore, wound healing following periodontal surgery was enhanced as a result of the antimicrobial effects of topically administered hydrogen peroxide.8
Bleaching agents are compounds that are used to remove color from substances, and most are oxidizing agents such as hydrogen peroxide, which are effective in decolorizing substances via oxidation. The decolorizing action of bleaches is due in part to their ability to remove electrons, which are activated by visible light to produce various colors.9 In 1966 Schneider, et al10 documented the use of a peroxide-containing gingival strip to apply peroxide to healing periodontal tissues. Tooth whitening was later observed as an unintentional side effect when hydrogen peroxide was used in periodontal treatment.11 In the late 1960s Klusmier noticed the whitening effect when using Gly-Oxide (GlaxoSmithKline) in orthodontic positioners.12 Later, Wagner used Proxigel (Reed and Carnrick Pharmaceutical) in custom-fitted vacuum-formed trays specifically for tooth whitening. These were FDA-approved oral antiseptics containing 10% carbamide peroxide.13
In 1989, Heymann and Haywood published a report that introduced the concept of using a nightguard with a viscous whitening solution that contained a thickening agent (Carbopol). The viscosity of the solution allowed for a longer bleach time and increased retention in the tray.14 In 1989 Fischer, who created Opalescence carbamide peroxide (Ultradent Products), received a patent for a thick and sticky whitening gel formulation that is still the basis for most night-time gels in use today. Carbamide peroxide breaks down into hydrogen peroxide and urea; hydrogen peroxide breaks down into oxygen and water; urea breaks down into ammonia and carbon dioxide. This was the first ADA-approved system for whitening.13 This product was developed with a high water content to minimize tooth sensitivity, a neutral pH, a thixotropic viscosity to improve retention in the tray, and sustained release of the hydrogen peroxide. With this introduction of home whitening, there has been explosive growth in consumer awareness and use of tooth-whitening systems. There are literally hundreds of competing products and delivery systems for in-office and home use. However, all systems employ the basic principles of exposing the discolored dentition to various forms of hydrogen peroxide over time.
The safety of hydrogen peroxide and carbamide peroxide has been documented in numerous studies. Haywood and Heymann15 evaluated vital bleaching using 10% carbamide peroxide in a nightguard, stating that concerns of toxicity or damage to hard and soft tissues appear unfounded. In a retrospective review of the medical and dental literature Yarborough16 states that the safety and efficacy of hydrogen peroxide is well-established. Hydrogen peroxide does not adversely affect enamel morphology or microhardness, and it is not expected to inhibit pulpal enzymes.17 Even when used for extended periods of time when treating tetracycline-stained teeth, no adverse effects have been noted using carbamide peroxide.18
EXTRINSIC AND INTRINSIC STAINS
 |
 |
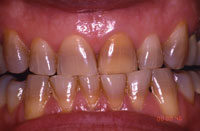
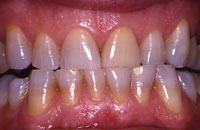
| Figure 1. A 45-year-old male demonstrating tetracycline staining and a nonvital tooth No. 9. (Figures 1 to 9 are courtesy of Dr. Bruce Matis.) |
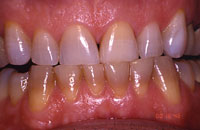
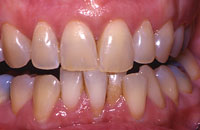
Figure 2. The patient after 4 months of take-home whitening. |
 |
|
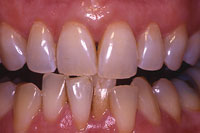
Figure 3. Three months post bleaching. |
Dental discoloration can be due to extrinsic staining, which is superficial and affects only the enamel surface. This type of discoloration is associated with the use of tea, coffee, chewing tobacco, some foods such as blueberries, and red wine. In addition, teeth discolor in association with aging. This type of discoloration is relatively easy to treat with tooth whitening. The intrinsic stains that discolor the tissues of the tooth (enamel, dentin), such as that related to fluorosis or tretracycline (Figures 1 to 3), are much more difficult to treat. Even though peroxides in whitening systems have been shown to rapidly permeate intact enamel, dentin, and pulp,19 changing the color of the dentin requires long exposure to produce any noticeable effect. Hydrogen peroxide releases oxygen that breaks down conjugated bonds in protein chains associated with stain into a single bond. This results in more absorption of color wavelengths, resulting in the reflection of little color (ie, a whitening effect).20
IN-OFFICE WHITENING
In-office tooth whitening is associated with a higher cost than take-home whitening systems due to chair time. In-office whitening is best for those patients who desire a quick result and for those who need close monitoring for clinical conditions such as pronounced gingival recession or deep, unrestored abfraction lesions. It is also necessary for tooth discoloration associated with endodontic therapy. In-office tooth whitening using high concentrations of hydrogen peroxide is not new. Boksman, et al published articles in the 1980s on the use of heated Superoxol (Sultan Healthcare) hydrogen peroxide (30%).21-24
Many current systems use light activation in conjunction with hydrogen peroxide. Examples include the following: LaserSmile (Biolase Technology) 37% hydrogen peroxide; ArcBrite (Biotrol) 30% hydrogen peroxide; Brite-Smile (BriteSmile) 15% hydrogen peroxide; Rembrandt Lightning Plus (Johnson & Johnson) 35% hydrogen peroxide; Zoom (Discus Dental) 20% hydrogen peroxide; and LumaWhite Plus (LumaLite) 35% hydrogen peroxide. Because of media coverage about light-activated bleaching, patient demand for this process is increasing. It is interesting to note that clinical trials repeatedly show that light and heat do not increase the efficacy of tooth whitening and are not necessary for vital tooth bleaching. Contact time and concentration of active ingredients are the critical factors.25
Light-activated whitening systems offer a marketing opportunity but add cost, occupy operatory space, can cause burning of the soft tissue, and can increase operatory temperature.26 All systems recommend a take-home tray as an adjunct, so the question is whether any observed benefit is due to the light or the tray.27 It is also important to note that many drugs patients may be using cause minor to marked photosensitivity and hyperpigmentation. These include acne medications, anticancer drugs, antidepressants, antihistamines, antimicrobials, anti-parasitic drugs, antipsychotic drugs, diuretics, hypogly-cemics, and nonsteroidal anti-inflammatory drugs.28 Therefore, the use of a light for in-office whitening may not be justified due to the risks involved.
 |
 |
|
Figure 4. Preoperative photograph of 46-year-old female requesting in-office whitening. |
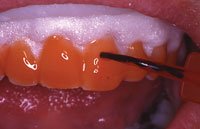
Figure 5. Opalescence Xtra Boost gel on teeth, with OpalDam (Ultra-dent Products) protecting the gingival tissues and covering the gingival margin of the tooth by 0.5 mm. |
 |
| Figure 6. After three, 15-minute applications of Opalescence Xtra Boost (at one appointment). |
In-office whitening systems not using light or heat include the following: Illumin with 15% hydrogen peroxide (DENTSPLY Professional) OfficeWhite with 40% hydrogen peroxide (Life-Like Cosmetic Solutions); Perfection White with 35% hydrogen peroxide (Premier Dental Products); Niveous with 25% hydrogen peroxide (Shofu Dental); and Opalescence Xtra Boost (Ultradent Products) with 38% hydrogen peroxide (Figures 4 to 6). Due to the adverse effects these high concentrations of hydrogen peroxide can have on the gingival tissues, many of these systems utilize various forms of tissue protection to minimize the potential for damage. The time of application and number of applications vary by product.
TAKE-HOME WHITENING
 |
 |
|
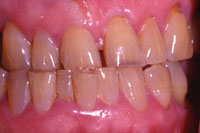
Figure 7. Prewhitening. |
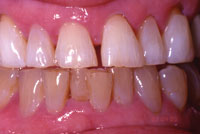
Figure 8. Same patient after whitening maxillary dentition with 10% Opalescence. |
 |
 |
|
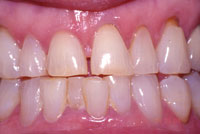
Figure 9. Same patient after whitening the mandibular arch. |
Figure 10. Trèswhite with 9% hydrogen peroxide gel and an outer gingival protector gel. |
At-home systems for tooth whitening, utilizing tray delivery of the whitening agent, have been extensively studied since their introduction by Heymann and Haywood14 (Figures 7 to 9). The degradation of carbamide peroxide occurs over time. After 2 hours, more than 50% of the active agent is available, and 10% is available after 10 hours.29 Therefore, for use at night, the maximum whitening effect occurs in the first 2 hours. Whitening agents that are recommended by their manufacturers for night-time use include the following: Opalescence PF (Ultradent Products) 10%, 15%, and 20% carbamide peroxide; Nupro White Gold (DENTSPLY Professional) 10% and 15% carbamide peroxide; Nite White Turbo (Discus Dental) 6% hydrogen peroxide; and Pola-Night (Southern Dental In-dustries) 10%, 16%, and 22% carbamide peroxide.
For daytime use, both carbamide peroxide and hydrogen peroxide are effective at-home bleaching agents.30 Products indicated by their manufacturers for daytime use include the following: Opalescence PF 10%, 15%, and 20% carbamide peroxide; Rembrandt XTRA-Comfort (Johnson & Johnson) 16%, 22%, and 30% carbamide peroxide; Natural Elegance (Henry Schein) 10%, 15%, and 22% carbamide peroxide; JustSmile (JustSmile Whitening Systems) 2% to 10% hydrogen peroxide; Perfecta Brav (Premier Dental Products) 9% hydrogen peroxide; and PolaDay (Southern Dental Industries) 3%, 7.5%, and 9.5% hydrogen peroxide.
 |
 |
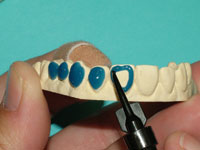
| Figure 11. Application of LC Block-Out Resin (Ultradent Products) on the labial surfaces of the teeth to be whitened. | Figure 12. Tray material in UltraVac Vacuum Former (Ultradent Products) showing a 2-inch drop of the tray material when heated sufficiently. |
 |
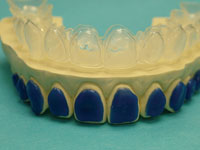 |
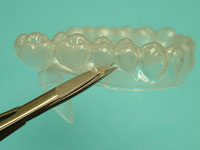
| Figure 13. Ultra-Trim Scalloping Scissors (Ultradent Products) scalloping the gingival margins to adapt precisely to the gingival margin. | Figure 14. Tray perfectly trimmed to the gingival margin, showing labial reservoir outline. |
With the take-home systems, custom trays are fabricated to trap the agent against the tooth surface. A reservoir created by placing a die spacer over the teeth can be created, or alternatively no die spacer is used. The reservoir technique creates a small space on the inside surface of the tray immediately adjacent to the buccal surface of the tooth. This space will trap a greater quantity of bleach than a nonreservoir technique. The increased bleach quantity will release more oxygen ions over a longer period of time in the vicinity of the tooth, creating a greater early whitening effect.36 Using colorimetric analysis, a study by Matis found that teeth lightened with trays containing a reservoir were lighter in color than teeth lightened with trays that did not have a reservoir. However, the difference was below the threshold of visual detection.37 It is also of importance to note that the amount of active material left after a period of time varies with tray design. In a study looking at the total carbamide peroxide percent recovered after timed use in various tray designs, Matis, et al demonstrated that the use of reservoirs resulted in a recovery of significantly higher carbamide peroxide after 2 hours.38 The use of reservoirs may or may not be necessary, with published data supporting both points of view39 (Figures 11 to 14).
Sales of over-the-counter whitening products have been estimated to approach $1 billion a year in North America alone.13 Companies (eg, Procter & Gamble, Colgate) offer whitening products whose effects have been documented.40,41 Crest Whitestrips (Procter & Gamble), containing a 6.5% hydrogen peroxide, were introduced in 2001. Many different versions of Colgate Platinum are available. Initially offered as whitening strips of various concentrations of hydrogen peroxide, a new delivery system utilizing a gel that is painted on the teeth (Colgate Simply-White Whitening Gel) containing 5.9% hydrogen peroxide has recently been introduced; it exceeds the ADA minimum requirements to claim clinical efficacy. 42
TOOTH SENSITIVITY
Transient tooth sensitivity occurring after whitening teeth with the products described in this article is dose and time dependent. The higher the dose or concentration of the whitening agent and the longer the teeth are exposed, the greater the risk of tooth sensitivity.43 If sensitivity occurs, the easiest way to address the problem is to decrease the time the patient treats the teeth or decrease the dosage of the peroxide or carbamide peroxide. Many products contain water to decrease the dehydration effects of whitening (Opalescence). Fluoride and potassium nitrate have been added to certain products such as Opalescence PF to decrease the incidence of tooth sensitivity. Potassium nitrate penetrates the dentinal tubules and depolarizes the nerves, decreasing the painful stimulus.44 Potassium nitrate gels, which can be used in bleaching-type trays to reduce hypersensitivity of root surfaces, include UltraEZ (Ultra-dent Products), Den-Mat Desensitize (Den-Mat), and Relief (Discus Dental). A preloaded tray version of UltraEZ is also available. Recently, amorphous calcium phosphate was added to products like Zoom2 (Discus Dental) to treat hypersensitivity.
CONCLUSION
Dental clinicians are in a unique position to play an active role in encouraging and educating dental patients regarding the choices that are available for tooth whitening. Tooth whitening is the easiest and least expensive of the treatment options that are available to change the shade of the dentition.
References
1. Burchard HH. A Textbook of Dental Pathology and Therapeutics. Philadelphia, Pa: Lea and Febiger; 1898.
2. Fitch CP. Etiology of the discoloration of teeth. Dental Cosmos. 1861;3:133-136.
3. Harlan AW. Hydrogen dioxide (in the treatment of alveolar abscess, pyorrhea and the bleaching of teeth). Dent Cosmos. 1882;24:515-523.
4. White JD. Bleaching. Dental Register of the West. 1861;15:576-577.
5. Wennstrom J, Lindhe J. Effect of hydrogen peroxide on developing plaque and gingivitis in man. J Clin Periodontol. 1979;6:115-130.
6. Volpe AR, Manhold JH, Manhold BS, et al. Gingival tissue oxygenation: the effect of a single application of four commercial preparations. J Periodontol. 1966;37:478-482.
7. Kelly TF. Hydrogen peroxide shows value of use. Dent Stud. 1976;54:66,82
8. Marshall MV, Cancro LP, Fischman SL. Hydrogen peroxide: a review of its use in dentistry. J Periodontol. 1995;66:786-796.
9. Asato R. Oxidation/reduction: bleaching agents. Kapií olani Community College Web site. Available at: http://library.kcc.hawaii.edu/external/chemistry/everyday_bleach.html. Accessed May 6, 2006.
10. Schneider HG, Birkholz C, Hampel W. Clinical experience with the peroxide-containing gingival strip from the Leipziger Arzneimttelwerk. Dtsch Stomatol. 1966;16:656-667.
11. Flaitz CM, Hicks MJ. Effects of carbamide peroxide whitening agents on enamel surfaces and caries-like lesion formation: an SEM and polarized light microscopic in vitro study. ASDC J Dent Child. 1996;63:249-256.
12. Goff S. Getting the white right. Dental Products Report. Jan 2005:14-19. Available at: http://www.dentalproducts.net/xml/display.asp?file=2716&bhcp=1. Accessed July 20, 2006.
13. Fasanaro TS. Bleaching teeth: history, chemicals, and methods used for common tooth discolorations. J Esthet Dent. 1992;4:71-78.
14. Haywood VB, Heymann HO. Night-guard vital bleaching. Quintessence Int. 1989;20:173-176.
15. Haywood VB, Heymann HO. Nightguard vital bleaching: how safe is it? Quintessence Int. 1991;22:515-523.
16. Yarborough DK. The safety and efficacy of tooth bleaching: a review of the literature 1988-1990. Compendium. 1991;12:191-196.
17. Pugh G Jr, Zaidel L, Lin N, et al. High levels of hydrogen peroxide in overnight tooth-whitening formulas: effects on enamel and pulp. J Esthet Restor Dent. 2005;17:40-47.
18. Haywood VB, Leonard RH, Dickinson GL. Efficacy of six months of nightguard vital bleaching of tetracycline-stained teeth. J Esthet Dent. 1997;9:13-19.
19. Cooper JS, Bokmeyer TJ, Bowles WH. Penetration of the pulp chamber by carbamide peroxide bleaching agents. J Endod. 1992;18(7):315-317.
20. Wade LG Jr. Conjugated systems. In: Organic Chemistry. 3rd ed. Upper Saddle River, NJ. Prentice Hall; 1994: chapter 15, 695, 1106.
21. Boksman L, Jordan RE. Conservative treatment of the stained dentition: vital bleaching. Aust Dent J. 1983;28:67-72.
22. Jordan RE, Suzuki M, Hunter JK, et al. Conservative treatment of the tetracycline stained dentition. Alpha Omegan. 1981;74:40-49.
23. Boksman L, Jordan RE, Skinner DH. A conservative bleaching treatment for the nonvital discolored tooth. Compend Contin Educ Dent. 1984;5:471-475.
24. Jordan RE, Boksman L. Conservative vital bleaching treatment of discolored dentition. Compend Contin Educ Dent. 1984;5:803-807.
25. CRA Newsletter. Issue 4, 2000.
26. CRA Newsletter. Issue 26, 2002.
27. Kugel G. Is there a benefit to light-activated tooth whitening? J Can Dent Assoc. 2005;71:420-421.
28. Drug-induced photosensitivity. Family Practice Notebook.com. Available at: http://www.fpnotebook.com/DER202.htm. Accessed May 6, 2006.
29. Matis BA, Gaiao U, Blackman D, et al. In vivo degradation of bleaching gel used in whitening teeth. J Am Dent Assoc. 1999;130:227-235.
30. Mokhlis GR, Matis BA, Cochran MA, et al. A clinical evaluation of carbamide peroxide and hydrogen peroxide whitening agents during daytime use. J Am Dent Assoc. 2000;131:1269-1277.
31. Araujo EM, Baratieri LN, Vieira LC, et al. In situ effect of 10% carbamide peroxide on microhardness of human enamel: function of time. J Esthet Restor Dent. 2003;15:166-173.
32. White DJ, Kozak KM, Zoladz JR, et al. Peroxide interactions with hard tissues: effects on surface hardness and surface/subsurface ultrastructural properties. Compend Contin Educ Dent. 2002;23:41-48.
33. Clark LM, Barghi N, Summitt JB, et al. Influence of fluoridated carbamide peroxide bleaching gel on enamel demineralization. Abstract 0497. International Association for Dental Research Web site. Available at: http://iadr.confex.com/iadr/2006Orld/techprogram/abstract_76079.html. Accessed May 2006.
34. Al-Qunaian T. The effect of whitening agents on caries susceptibility of human enamel. Oper Dent. 2005;30:265-270.
35. Basting RT, Rodrigues AL Jr, Serra MC. The effects of seven carbamide peroxide bleaching agents on enamel microhardness over time. J Am Dent Assoc. 2003;134:1335-1342.
36. Buyersí guide to whitening systems. Dent Today. Dec 2004;23(12):120.
37. Matis BA, Hamdan YS, Cochran MA, et al. A clinical evaluation of a bleaching agent used with and without reservoirs. Oper Dent. 2002;27:5-11.
38. Matis BA, Yousef M, Cochran MA, et al. Degradation of bleaching gels in vivo as a function of tray design and carbamide peroxide concentration. Oper Dent. 2002;27:12-18.
39. Javaheri DS, Janis JN. The efficacy of reservoirs in bleaching trays. Oper Dent. 2000;25:149-151.
40. Gerlach RW, Zhou X. Clinical trial comparing two daytime hydrogen-peroxide professional vital-bleaching systems. Compend Contin Educ Dent. 2004;25(8 suppl 2):33-40.
41. Sagel PA, Landrigan WF. A new approach to strip-based tooth whitening: 14% hydrogen peroxide delivered via controlled low dose. Compend Contin Educ Dent. 2004;25(8 suppl 2):9-13.
42. Gambarini G, Testarelli L, De Luca M, et al. Efficacy and safety assessment of a new liquid tooth whitening gel containing 5.9% hydrogen peroxide. Am J Dent. 2004;17:75-79.
43. Boksman L. A large proportion of the patients who undergo teeth whitening procedures in my office experience sensitivity. How can I minimize this side effect? J Can Dent Assoc. Dec 2005/Jan 2006;71:829-830.
44. Orchardson R, Gillam DG. The efficacy of potassium salts as agents for treating dentin hypersensitivity. J Orofac Pain. 2000;14:9-19.
Dr. Boksman is adjunct clinical professor at the Schulich School of Medicine and Dentistry, University of Western Ontario. He is a fellow in the Academy of Dentistry International and in the International College of Dentists. He can be reached at (519) 641-3066, extension 292, or lboksman@clinicianschoice.com.
Disclosure: Dr. Boksman holds a paid part-time consulting position with Clinicians Choice and Clinical Research Dental, with the title of director of clinical affairs.
|
|
Continuing Education Test No. 81.1 |
|
After reading this article, the individual will learn:
ï about the development of bleaching agents, and
ï how to choose the appropriate tooth-whitening system for each patient.
1. Hydrogen peroxide in dentistry ____.
a. has been in use for more than 100 years
b. has been found to promote bone healing
c. has no antimicrobial effects
d. cannot be used in conjunction with periodontal surgery
2. The carbamide peroxide reaction is defined as follows:
a. Carbamide peroxide degrades into hydrogen peroxide and water.
b. Carbamide peroxide degrades into oxygen and water.
c. Carbamide peroxide degrades into ammonia and carbon dioxide.
d. Carbamide peroxide degrades into hydrogen peroxide and urea.
3. Hydrogen peroxide in all whitening agents _____.
a. is toxic to soft tissues
b. is safe to use
c. is detrimental to the pulp
d. decreases the microhardness of enamel
4. Which of the following is not an intrinsic stain?
a. nicotine staining
b. fluorosis
c. amalgam staining
d. tetracycline
5. In-office tooth whitening is best for ___.
a. patients with time limitations
b. patients who need close monitoring
c. teeth that have been endodontically treated
d. all of the above
6. For in-office tooth whitening, _____.
a. heat increases the whitening effect
b. light increases the whitening effect
c. concentration is the only critical factor that determines the degree of whitening
d. contact time and concentration of active ingredients are the critical factors
7. Hydrogen peroxide _____.
a. increases enamel’s susceptibility to develop caries
b. decreases the microhardness of enamel
c. in Opalescence PF decreases susceptibility for caries
d. with fluoride added is not absorbed into enamel
8. Tooth sensitivity during whitening ____.
a. can be treated by decreasing dosage and time of whitening
b. can be treated with potassium nitrate to depolarize nerves
c. can be reduced by using agents that contain water
d. all of the above
To submit Continuing Education answers, download the answer sheet in PDF format (click Download Now button below). Print the answer sheet, identify the article (this one is Test 81.1), place an X in the box corresponding to the answer you believe is correct, and mail to Dentistry Today Department of Continuing Education (complete address is on the answer sheet).